Home /
Expert Answers /
Chemistry /
calculate-the-entropy-of-the-following-reaction-in-mathrm-j-mathrm-k-using-the-ent-pa954
(Solved): Calculate the entropy of the following reaction (in \( \mathrm{J} / \mathrm{K} \) ), using the ent ...
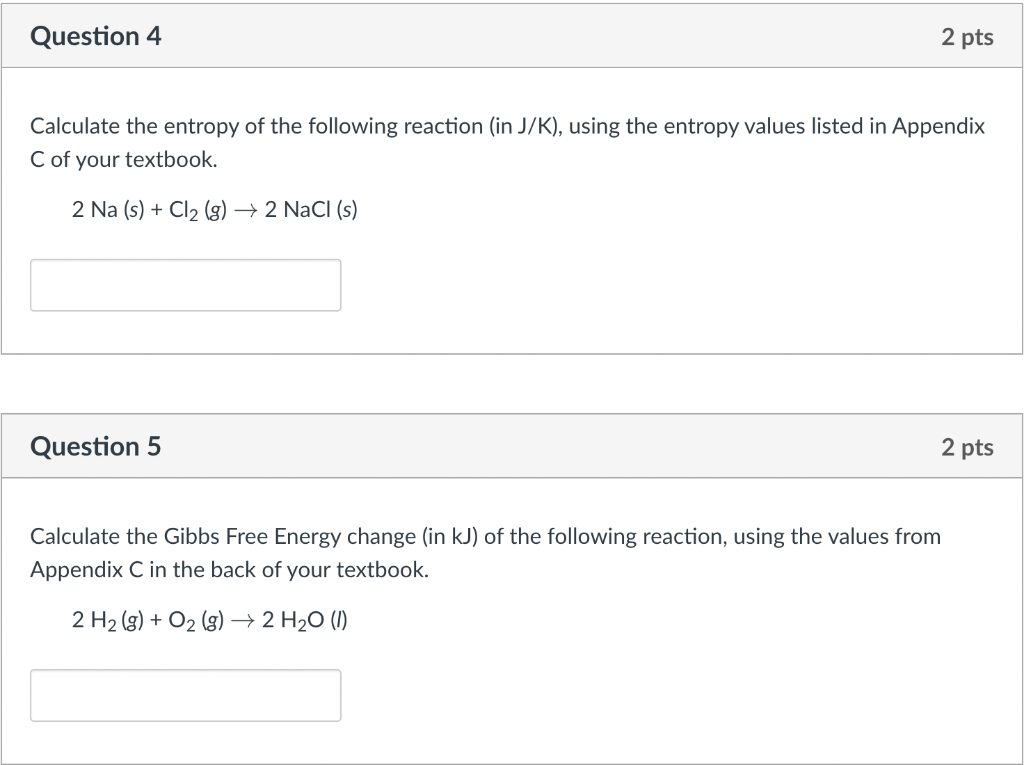
Calculate the entropy of the following reaction (in \( \mathrm{J} / \mathrm{K} \) ), using the entropy values listed in Appendix C of your textbook. \[ 2 \mathrm{Na}(\mathrm{s})+\mathrm{Cl}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{NaCl}(\mathrm{s}) \] Question 5 2 pts Calculate the Gibbs Free Energy change (in \( \mathrm{kJ} \) ) of the following reaction, using the values from Appendix \( \mathrm{C} \) in the back of your textbook. \[ 2 \mathrm{H}_{2}(g)+\mathrm{O}_{2}(g) \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\text { I }) \]