Home /
Expert Answers /
Chemistry /
electrically-neutral-atoms-the-boxes-below-are-labeled-with-the-approximate-atomic-masses-of-four-di-pa921
(Solved): Electrically neutral atoms The boxes below are labeled with the approximate atomic masses of four di ...
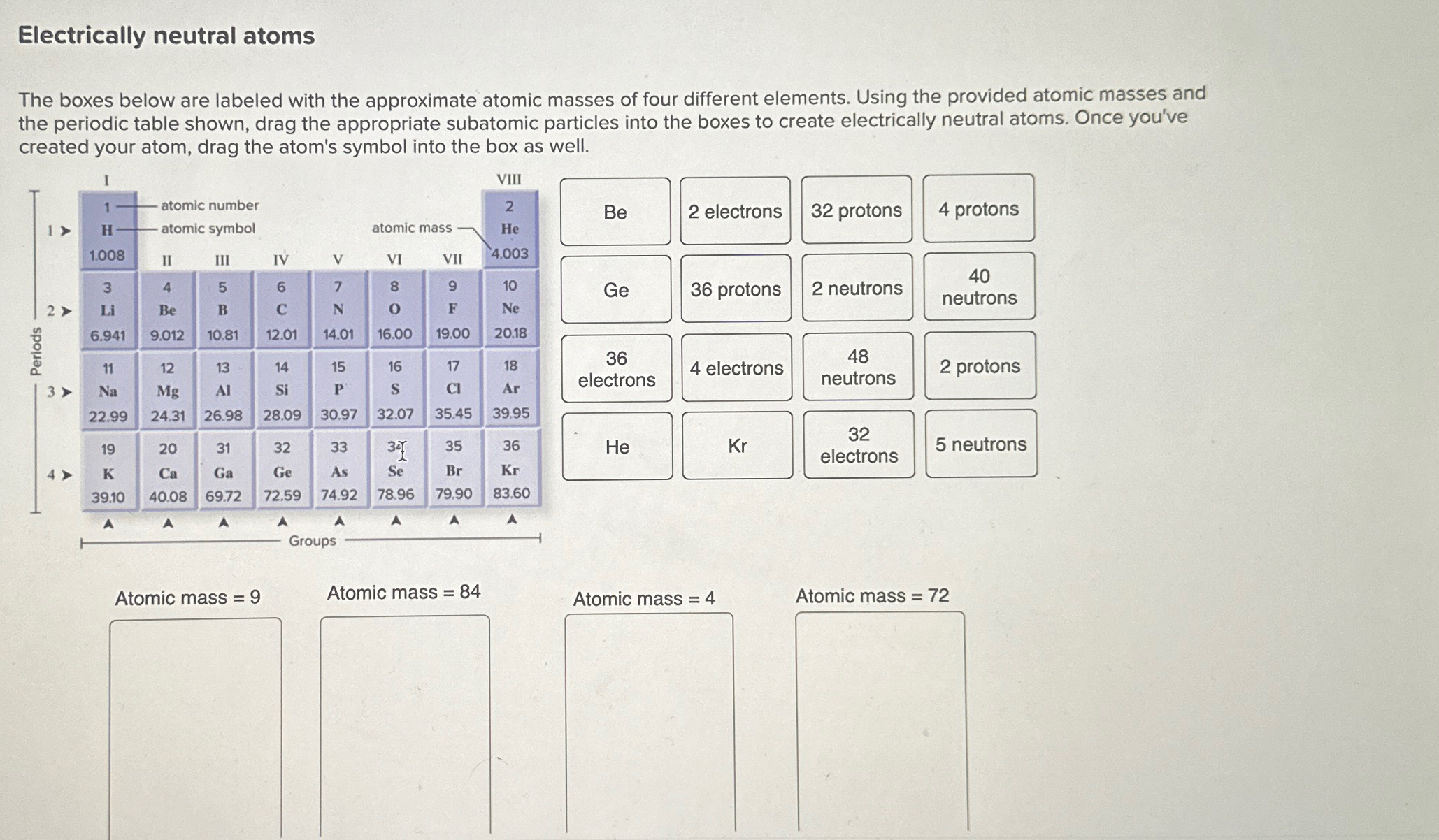
Electrically neutral atoms The boxes below are labeled with the approximate atomic masses of four different elements. Using the provided atomic masses and the periodic table shown, drag the appropriate subatomic particles into the boxes to create electrically neutral atoms. Once you've created your atom, drag the atom's symbol into the box as well. \table[[
Be,2 electrons,32 protons,4 protons],[
Ge,36 protons,2 neutrons,\table[[40],[neutrons]]],[\table[[36],[electrons]],4 electrons,\table[[48],[neutrons]],2 protons],[
He,
Kr,\table[[32],[electrons]],5 neutrons]] Atomic mass
=9Atomic mass
=84Atomic mass
=72
?