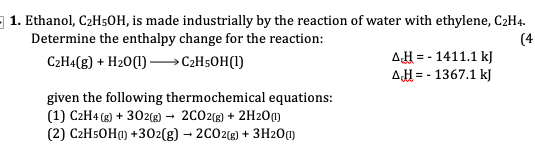
(Solved): Ethanol, C_(2)H_(5)OH, is made industrially by the reaction of water with ethylene, C_(2)H_(4). De ...
Ethanol,
C_(2)H_(5)OH, is made industrially by the reaction of water with ethylene,
C_(2)H_(4). Determine the enthalpy change for the reaction:
C_(2)H_(4)(g)+H_(2)O(l)longrightarrowC_(2)H_(5)OH(l)
\Delta _(c)H=-1411.1kJ
\Delta _(c)H=-1367.1kJgiven the following thermochemical equations: (1)
C_(2)H_(4)(g)
+3O_(2)(g)
->2CO_(2)(g)
+2H_(2)O_((l))(2)
C_(2)H_(5)OH(l)+3O_(2)(g)->2CO_(2)(g)+3H_(2)O_((l))1. Ethanol, C2H5OH, is made industrially by the reaction of water with ethylene, C2H4. Determine the enthalpy change for the reaction: C2H4(g) + H2O(l) C2H5OH(l) given the following thermochemical equations: (1) C2H4 (g) + 3O2(g) -> 2CO2(g) + 2H2O(l) ?rH = - 1411.1 kJ (2) C2H5OH(l) +3O2(g) -> 2CO2(g) + 3H2O(l) ?rH= - 1367.1 kJ 2. Consider the following reaction equation of combustion of glucose, C6H12O6(s) C6H12O6(s) + 6O2(g) -> 6CO2(g) + 6H2O(g) a. Calculate enthalpy of combustion of glucose, C6H12O6(s), b. Calculate molar enthalpy of combustion of glucose, C6H12O6(s), c. Calculate the enthalpy change when 5.25 g of glucose undergoes combustion.