Home /
Expert Answers /
Chemistry /
in-which-direction-will-the-reaction-proceed-consider-the-reaction-and-its-equilibrium-constant-at-pa167
(Solved): In which direction will the reaction proceed? Consider the reaction and its equilibrium constant at ...
In which direction will the reaction proceed?
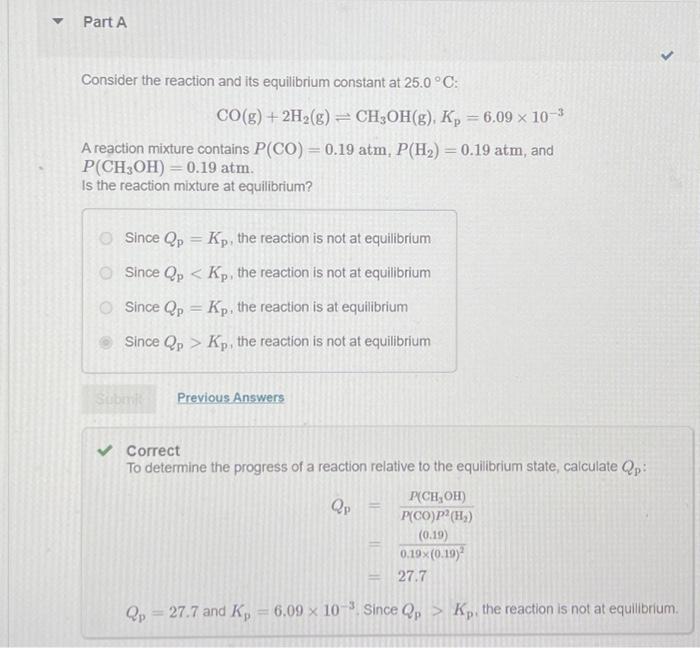

Consider the reaction and its equilibrium constant at : A reaction mixture contains , and atm. is the reaction mixture at equilibrium? Since , the reaction is not at equilibrium Since , the reaction is not at equilibrium Since , the reaction is at equilibrium Since , the reaction is not at equilibrium Correct To determine the progress of a reaction relative to the equilibrium state, calculate : and since the reaction is not at equilibrium
If not, in which direction will the reaction proceed? Check all that apply. in the reverse direction to the left to the right in the forward direction