Home /
Expert Answers /
Chemistry /
predictions-based-reduction-potentials-determine-the-spontaneity-consider-the-standard-reduction-p-pa360
(Solved): Predictions Based Reduction Potentials Determine the spontaneity Consider the standard reduction p ...
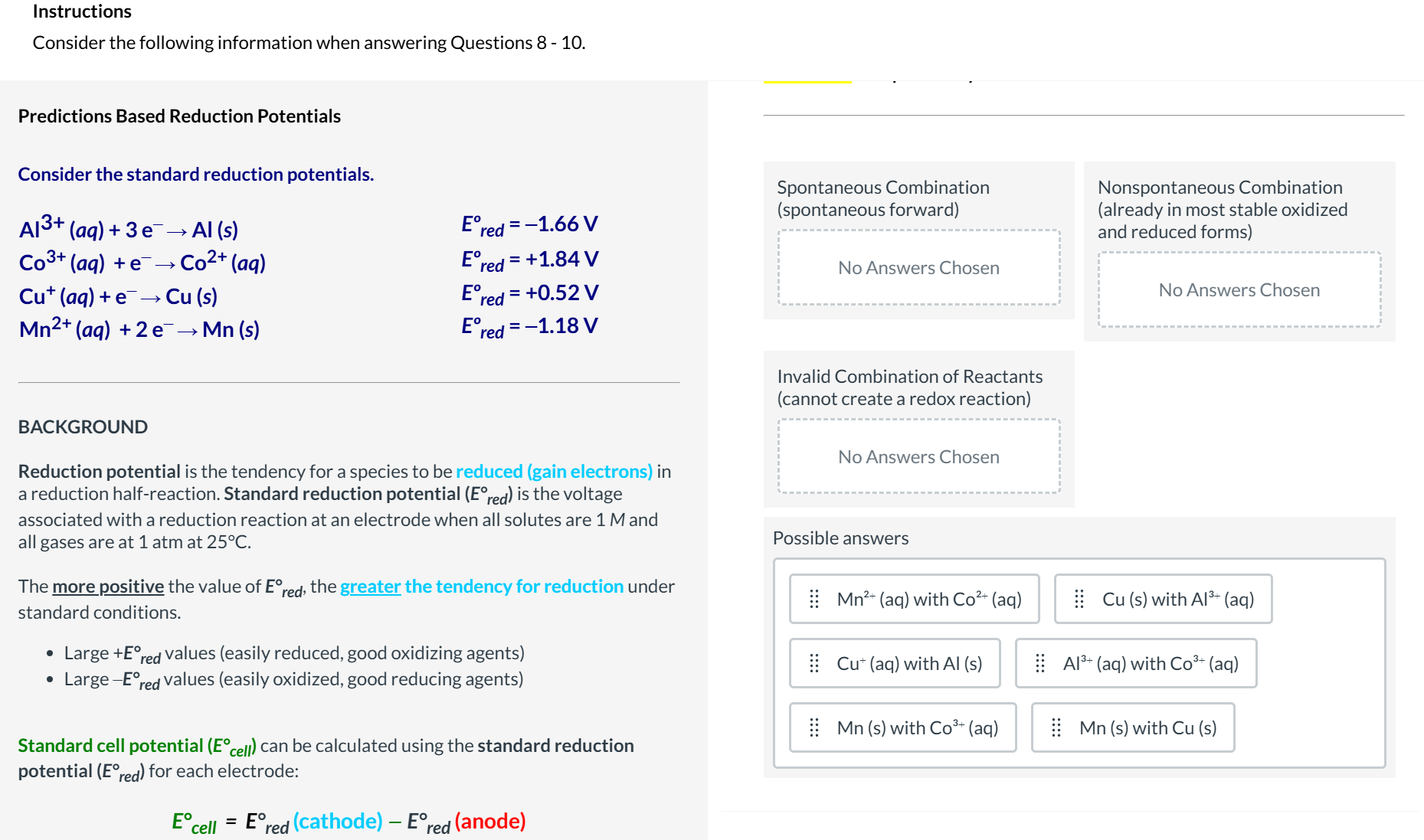
Predictions Based Reduction Potentials
Determine the spontaneity
Consider the standard reduction potentials.
Al^(3+)(aq)+3e^(-)->Al(s)
Co^(3+)(aq)+e^(-)->Co^(2+)(aq)
Cu^(+)(aq)+e^(-)->Cu(s)
Mn^(2+)(aq)+2e^(-)->Mn(s)
E_(red )^(o)=-1.66V
E_(red )^(o)=+1.84V
E_(red )^(o)=+0.52V
E_(red )^(o)=-1.18VE_(red )\deg 25\deg C.
The more positive the value of E_(red )\deg , the greater the tendency for reduction under
standard conditions.
Large +E_(red )\deg values (easily reduced, good oxidizing agents)
Large -E_(red )\deg E\deg cell