Home /
Expert Answers /
Chemistry /
q1-calculate-the-total-numbers-of-metal-bonds-in-the-following-compounds-10-marks-co-2-co-8-pa613
(Solved): Q1: Calculate the total numbers of Metal bonds in the following compounds. (10 marks) Co_(2)(CO)_(8) ...
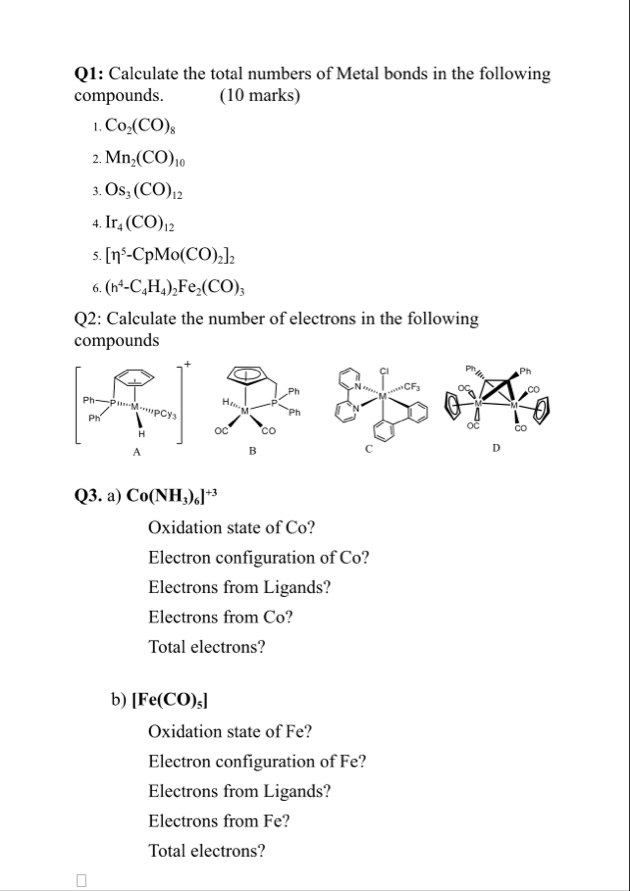
Q1: Calculate the total numbers of Metal bonds in the following compounds. (10 marks)
Co_(2)(CO)_(8)
Mn_(2)(CO)_(10)
Os_(3)(CO)_(12)
Ir_(4)(CO)_(12)
[\eta ^(5)-CpMo(CO)_(2)]_(2)
(^(4)-C_(4)H_(4))_(2)Fe_(2)(CO)_(3)Q2: Calculate the number of electrons in the following compounds Q3. a)
Co(NH_(3))_(6)Oxidation state of Co ? Electron configuration of Co ? Electrons from Ligands? Electrons from Co? Total electrons? b)
Fe(CO)_(5)Oxidation state of Fe ? Electron configuration of Fe ? Electrons from Ligands? Electrons from Fe ? Total electrons?