Home /
Expert Answers /
Chemistry /
using-data-from-the-thermodynamic-data-table-calculate-delta-g-in-mathrm-kj-pa418
(Solved): Using data from the Thermodynamic Data table, calculate \\( \\Delta G \\) (in \\( \\mathrm{kJ} \\) ...
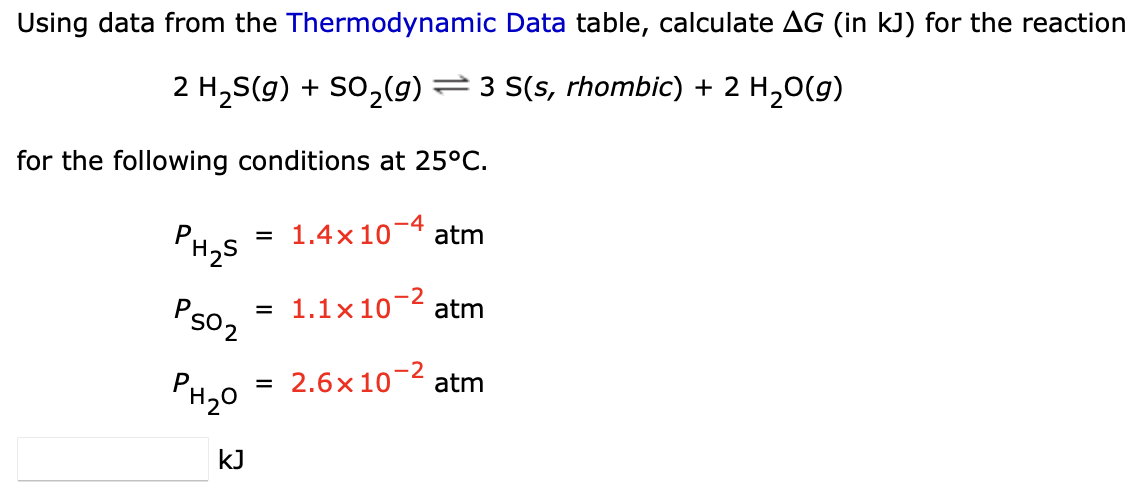
Using data from the Thermodynamic Data table, calculate \\( \\Delta G \\) (in \\( \\mathrm{kJ} \\) ) for the reaction \\[ 2 \\mathrm{H}_{2} \\mathrm{~S}(g)+\\mathrm{SO}_{2}(g) \\rightleftharpoons 3 \\mathrm{~S}(s, \\text { rhombic })+2 \\mathrm{H}_{2} \\mathrm{O}(g) \\] for the following conditions at \\( 25^{\\circ} \\mathrm{C} \\). \\[ \\begin{array}{l} P_{\\mathrm{H}_{2} \\mathrm{~S}}=1.4 \\times 10^{-4} \\mathrm{~atm} \\\\ P_{\\mathrm{SO}_{2}}=1.1 \\times 10^{-2} \\mathrm{~atm} \\\\ P_{\\mathrm{H}_{2} \\mathrm{O}}=2.6 \\times 10^{-2} \\mathrm{~atm} \\\\ \\mathrm{~kJ} \\end{array} \\]
Expert Answer
The given reaction is: The partial pressure of gasous species is given Using partial pressur...