Home /
Expert Answers /
Chemical Engineering /
1-a-calculate-the-value-of-standard-molar-enthalpy-and-entropy-of-the-chemi-pa327
(Solved): 1. a) Calculate the value of standard molar enthalpy and entropy of the chemi ...
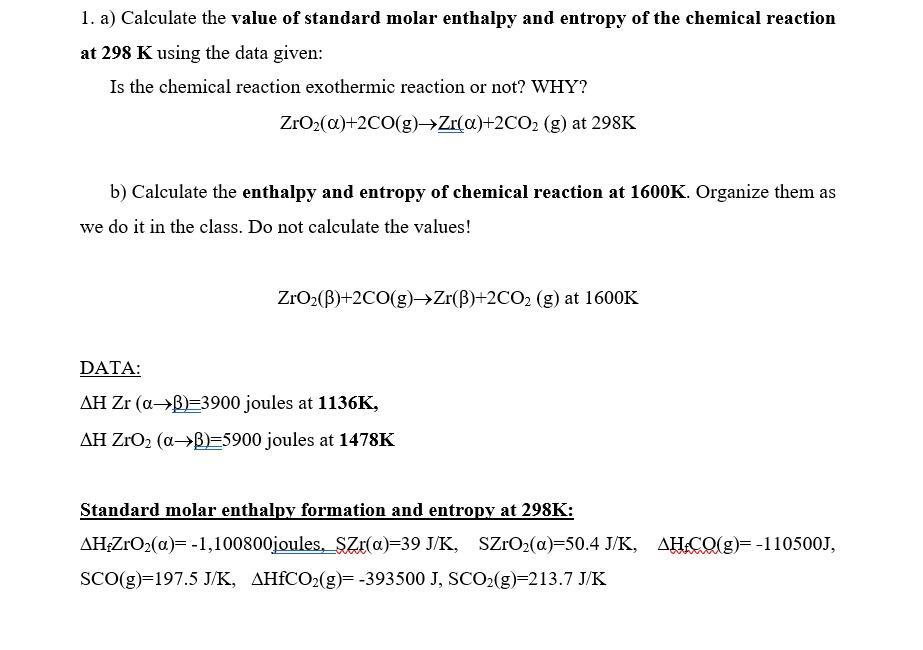
1. a) Calculate the value of standard molar enthalpy and entropy of the chemical reaction at \( 298 \mathrm{~K} \) using the data given: Is the chemical reaction exothermic reaction or not? WHY? \[ \mathrm{ZrO}_{2}(\alpha)+2 \mathrm{CO}(\mathrm{g}) \rightarrow \underline{\mathrm{Zr}}(\alpha)+2 \mathrm{CO}_{2}(\mathrm{~g}) \text { at } 298 \mathrm{~K} \] b) Calculate the enthalpy and entropy of chemical reaction at \( 1600 \mathrm{~K} \). Organize them as we do it in the class. Do not calculate the values! \[ \mathrm{ZrO}_{2}(\beta)+2 \mathrm{CO}(\mathrm{g}) \rightarrow \mathrm{Zr}(\beta)+2 \mathrm{CO}_{2}(\mathrm{~g}) \text { at } 1600 \mathrm{~K} \] DATA: \( \Delta \mathrm{H} \mathrm{Zr}(\alpha \rightarrow \beta)=3900 \) joules at \( 1136 \mathrm{~K} \), \( \Delta \mathrm{H} \mathrm{ZrO}_{2}(\alpha \rightarrow \beta)=5900 \) joules at \( \mathbf{1 4 7 8 K} \) Standard molar enthalpy formation and entropy at 298K: \[ \Delta \mathrm{H}_{\mathrm{f}} \mathrm{ZrO}_{2}(\alpha)=-1,100800 \text { joules, } \mathrm{SZr}(\alpha)=39 \mathrm{~J} / \mathrm{K}, \quad \mathrm{SZrO}_{2}(\alpha)=50.4 \mathrm{~J} / \mathrm{K}, \quad \Delta \mathrm{H}_{1} \mathrm{CO}(\mathrm{g})=-110500 \mathrm{~J} \text {, } \] \[ \mathrm{SCO}(\mathrm{g})=197.5 \mathrm{~J} / \mathrm{K}, \quad \Delta \mathrm{HfCO}_{2}(\mathrm{~g})=-393500 \mathrm{~J}, \mathrm{SCO}_{2}(\mathrm{~g})=213.7 \mathrm{~J} / \mathrm{K} \]
Expert Answer
To calculate the standard molar enthalpy of the chemical reaction at 298 K, we need to use the standard molar enthalpies of formation for the reactants and products. The standard molar entha