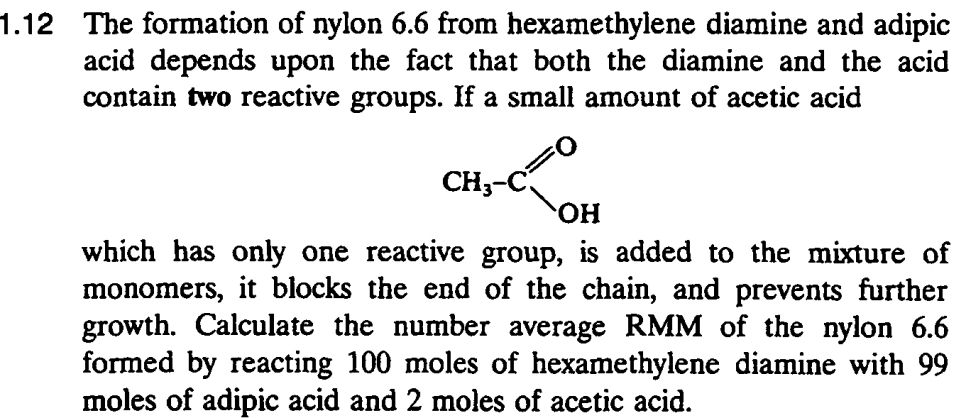
(Solved): .12 The formation of nylon 6.6 from hexamethylene diamine and adipic acid depends upon the fact that ...
.12 The formation of nylon 6.6 from hexamethylene diamine and adipic acid depends upon the fact that both the diamine and the acid contain two reactive groups. If a small amount of acetic acid D. 24 COM CH3-C OH which has only one reactive group, is added to the mixture of monomers, it blocks the end of the chain, and prevents further growth. Calculate the number average RMM of the nylon 6.6 formed by reacting 100 moles of hexamethylene diamine with 99 moles of adipic acid and 2 moles of acetic acid. tom fram nolvonta- 1.12 The formation of nylon 6.6 from hexamethylene diamine and adipic acid depends upon the fact that both the diamine and the acid contain two reactive groups. If a small amount of acetic acid which has only one reactive group, is added to the mixture of monomers, it blocks the end of the chain, and prevents further growth. Calculate the number average RMM of the nylon 6.6 formed by reacting 100 moles of hexamethylene diamine with 99 moles of adipic acid and 2 moles of acetic acid.