Home /
Expert Answers /
Chemistry /
2-pyrrole-is-highly-activated-towards-eas-reactions-compared-to-a-regular-benzene-ring-please-just-pa645
(Solved): 2. Pyrrole is highly activated towards EAS reactions compared to a regular benzene ring. Please just ...
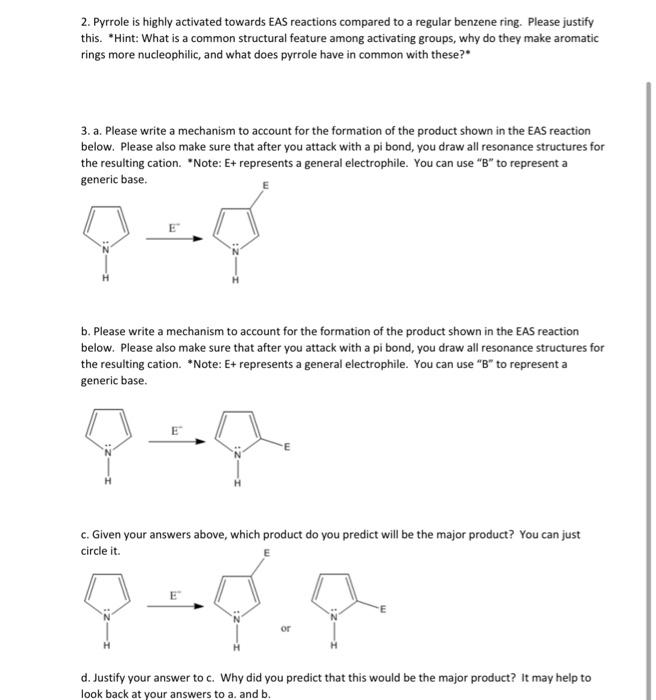
2. Pyrrole is highly activated towards EAS reactions compared to a regular benzene ring. Please justify
this. *Hint: What is a common structural feature among activating groups, why do they make aromatic
rings more nucleophilic, and what does pyrrole have in common with these?*
3. a. Please write a mechanism to account for the formation of the product shown in the EAS reaction
below. Please also make sure that after you attack with a pi bond, you draw all resonance structures for
the resulting cation. *Note: E+ represents a general electrophile. You can use “B” to represent a
generic base.
b. Please write a mechanism to account for the formation of the product shown in the EAS reaction
below. Please also make sure that after you attack with a pi bond, you draw all resonance structures for
the resulting cation. *Note: E+ represents a general electrophile. You can use “B” to represent a
generic base.
c. Given your answers above, which product do you predict will be the major product? You can just
circle it.
d. Justify your answer to c. Why did you predict that this would be the major product? It may help to
look back at your answers to a. and b
PLEASE ANSWER ALL PARTS AND PLEASE SHOW ALL RESONANCE STRUCTURES FOR THE RESULTING CATION AFTER ATTACKING WITH A PI BOND
2. Pyrrole is highly activated towards EAS reactions compared to a regular benzene ring. Please justify this. "Hint: What is a common structural feature among activating groups, why do they make aromatic rings more nucleophilic, and what does pyrrole have in common with these?* 3. a. Please write a mechanism to account for the formation of the product shown in the EAS reaction below. Please also make sure that after you attack with a pi bond, you draw all resonance structures for the resulting cation. "Note: \( \mathrm{E}+ \) represents a general electrophile. You can use "B" to represent a generic base. b. Please write a mechanism to account for the formation of the product shown in the EAS reaction below. Please also make sure that after you attack with a pi bond, you draw all resonance structures for the resulting cation. *Note: \( \mathrm{E}+ \) represents a general electrophile. You can use "B" to represent a generic base. c. Given your answers above, which product do you predict will be the major product? You can just d. Justify your answer to \( \mathrm{c} \). Why did you predict that this would be the major product? It may help to look back at your answers to \( a \). and \( b \).
Expert Answer
The internmediate formed by attack of the nucleophil