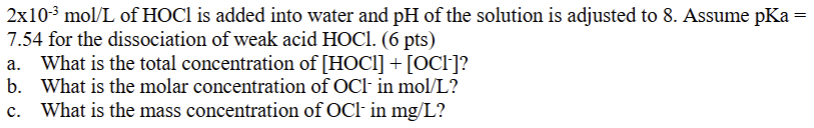Home /
Expert Answers /
Chemistry /
2-times-10-3-mo-l-l-of-hocl-is-added-into-water-and-ph-of-the-solution-is-adjusted-to-8-assume-pa714
(Solved): 2\times 10^(-3)mo(l)/(L) of HOCl is added into water and pH of the solution is adjusted to 8. Assume ...
2\times 10^(-3)mo(l)/(L) of HOCl is added into water and pH of the solution is adjusted to 8. Assume pKa = 7.54 for the dissociation of weak acid HOCl . a. What is the total concentration of [HOCl]+[OCl^(-)]? b. What is the molar concentration of OCl^(-)in mo(l)/(L) ? c. What is the mass concentration of OCl^(-)in m(g)/(L) ?