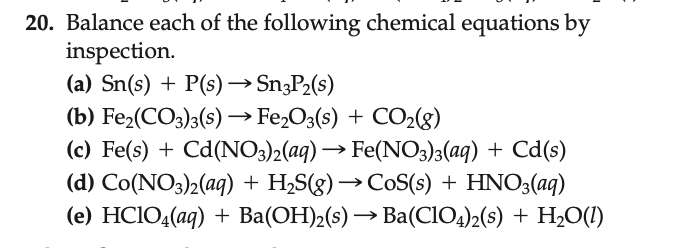Home /
Expert Answers /
Chemistry /
20-balance-each-of-the-following-chemical-equations-by-inspection-a-sn-s-p-s-sn3p2-s-pa564
Expert Answer
Balancing a chemical equation involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is done by placing coefficients in front of the chemical formulas where necessary. Let's balance each of these equations:(a) Sn(s) + P(s) ? Sn3P2(s) Balance the Sn (tin) and P (phosphorus) atoms: This reaction involves tin (Sn) and phosphorus (P) to form tin phosphide (Sn3P2).The coefficient 3 in front of Sn and 2 in front of P ensures the same number of tin and phosphorus atoms on both sides of the equation.Tin and phosphorus atoms are reacting in a 3:2 ratio to produce tin phosphide.(b) Fe2(CO3)3(s) ? Fe2O3(s) + CO2(g) Balance the Fe (iron), C (carbon), and O (oxygen) atoms: This reaction involves iron carbonate (Fe2(CO3)3) decomposing into iron (III) oxide (Fe2O3) and carbon dioxide (CO2).The coefficient 3 in front of CO2 ensures the same number of carbon and oxygen atoms on both sides of the equation.The iron carbonate is decomposing in a 1:1:3 ratio to produce iron oxide and carbon dioxide.