Home /
Expert Answers /
Chemistry /
22-complete-table-1-below-you-do-not-need-to-show-your-work-write-a-point-form-summary-of-the-pa240
(Solved): 22. Complete Table 1 (below). You do not need to show your work. Write a point form summary of the ...
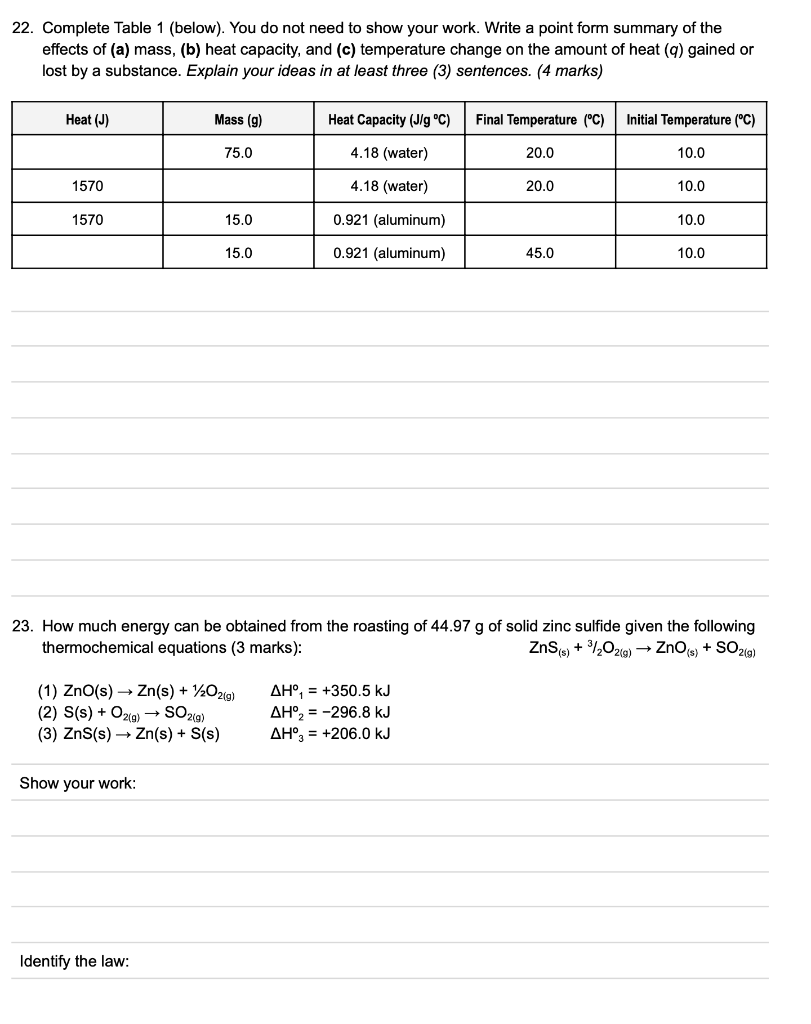
22. Complete Table 1 (below). You do not need to show your work. Write a point form summary of the effects of (a) mass, (b) heat capacity, and (c) temperature change on the amount of heat ( \( q) \) gained or lost by a substance. Explain your ideas in at least three (3) sentences. (4 marks) 23. How much energy can be obtained from the roasting of \( 44.97 \mathrm{~g} \) of solid zinc sulfide given the following thermochemical equations ( 3 marks): \[ \mathrm{ZnS}_{(\mathrm{s})}+3 /{ }_{2} \mathrm{O}_{2(g)} \rightarrow \mathrm{ZnO}_{(\mathrm{s})}+\mathrm{SO}_{2(g)} \] (1) \( \mathrm{ZnO}(\mathrm{s}) \rightarrow \mathrm{Zn}(\mathrm{s})+1 / 2 \mathrm{O}_{2(g)} \) \( \Delta \mathrm{H}^{\circ}{ }_{1}=+350.5 \mathrm{~kJ} \) (2) \( \mathrm{S}(\mathrm{s})+\mathrm{O}_{2(g)} \rightarrow \mathrm{SO}_{2(g)} \) \( \Delta \mathrm{H}_{2}^{\circ}=-296.8 \mathrm{~kJ} \) (3) \( \mathrm{ZnS}(\mathrm{s}) \rightarrow \mathrm{Zn}(\mathrm{s})+\mathrm{S}(\mathrm{s}) \) \( \Delta \mathrm{H}^{\circ}{ }_{3}=+206.0 \mathrm{~kJ} \) Show your work: Identify the law:
Expert Answer
As per Chegg guidelines, only 1st question can be answered, for the remaining questions post them separately. Answer to your 22nd question is given below: Solution: Step 1: In specific heat formula, the mass and temperature change are directly propor