Home /
Expert Answers /
Mechanical Engineering /
25-q1-the-piston-of-a-frictionless-piston-cylinder-apparatus-shown-in-figure-q1-is-fixed-wit-pa171
(Solved): (25%) Q1. The piston of a frictionless piston-cylinder apparatus shown in Figure Q1 is fixed wit ...
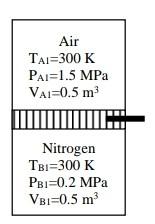
(25%) Q1. The piston of a frictionless piston-cylinder apparatus shown in
Figure Q1 is fixed with a pin at a location dividing the cylinder
into two equal volumes of 0.5 m³. One of the compartments is
filled with air at a temperature of 300 K and pressure of 1.5
MPa, and the other is nitrogen at 300 K and 0.2 MPa.
Suddenly the pin fixing the piston is set free and the system
reaches a temperature of 300 K when the thermodynamic
equilibrium is established. Find the mass, pressure and volume
of each gas at the equilibrium state.
Air
TAI 300 K
PAI=1.5 MPa
VAI=0.5 m³
IH
Nitrogen
TBI=300 K
PBI=0.2 MPa
VBI=0.5 m³
Fig. Ql
\begin{tabular}{|c|} \hline \begin{array}{l} Air \\ \( \mathrm{T}_{\mathrm{Al}}=300 \mathrm{~K} \\ \mathrm{P}_{\mathrm{Al}}=1.5 \mathrm{MPa} \\ \mathrm{V}_{\mathrm{Al}}=0.5 \mathrm{~m}^{3}\end{array} \) \\ \hline \\ \hline \begin{aligned} Nitrogen & \\ \( \mathrm{T}_{\mathrm{B} 1} & =300 \mathrm{~K} \\ \mathrm{P}_{\mathrm{B} 1} & =0.2 \mathrm{MPa} \\ \mathrm{V}_{\mathrm{B} 1} & =0.5 \mathrm{~m}^{3}\end{aligned} \) \\ \hline \end{tabular}