Home /
Expert Answers /
Chemistry /
3-7-points-consider-the-pentadienyl-catio-a-how-many-electrons-are-in-the-pi-system-b-pa219
(Solved): 3. (7 points) Consider the pentadienyl catio a. How many electrons are in the \( \pi \) system? b. ...
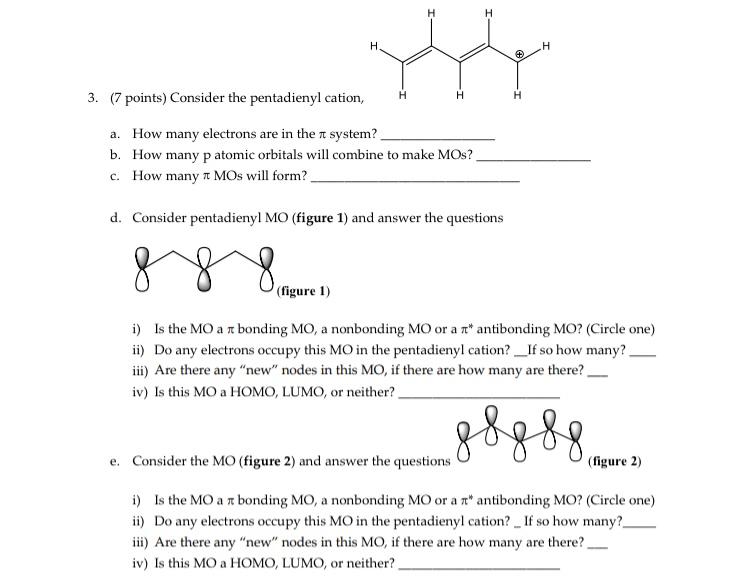
3. (7 points) Consider the pentadienyl catio a. How many electrons are in the \( \pi \) system? b. How many p atomic orbitals will combine to make MOs? c. How many \( \pi \mathrm{MOs} \) will form? d. Consider pentadienyl MO (figure 1) and answer the questions i) Is the MO a \( \pi \) bonding MO, a nonbonding MO or a \( \pi^{*} \) antibonding MO? (Circle one) ii) Do any electrons occupy this \( \mathrm{MO} \) in the pentadienyl cation? _If so how many? iii) Are there any "new" nodes in this MO, if there are how many are there? iv) Is this MO a HOMO, LUMO, or neither? e. Consider the MO (figure 2) and answer the questions (figure 2) i) Is the MO a \( \pi \) bonding MO, a nonbonding MO or a \( \pi^{*} \) antibonding MO? (Circle one) ii) Do any electrons occupy this MO in the pentadienyl cation? _ If so how many? iii) Are there any "new" nodes in this MO, if there are how many are there? iv) Is this MO a HOMO, LUMO, or neither?