Home /
Expert Answers /
Chemistry /
3-93-when-heated-lithium-reacts-with-nitrogen-to-form-lithium-nitride-6li-s-n2-g-2li3-pa964
(Solved): 3.93 When heated, lithium reacts with nitrogen to form lithium nitride: 6Li(s)+N2(g)2Li3 ...
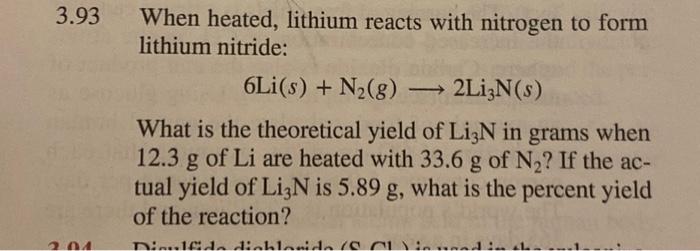
3.93 When heated, lithium reacts with nitrogen to form lithium nitride: What is the theoretical yield of in grams when of are heated with of ? If the actual yield of is , what is the percent yield of the reaction?
Expert Answer
To determine the theoretical yield of and the percent yield of the reaction, we'll follow these steps: Step 1: Calculate the molar masses of Li and N?: Molar mass of Li = 6.94 g/mol Molar mass of N? = 28.02 g/molStep 2: Determine the limiting reactant: To determine the limiting reactant, we compare the mole ratios of Li and N? in the balanced equation to the actual amounts given. From the balanced equation, the mole ratio of Li to is 6:2, and the mole ratio of N? to The number of moles of Li: moles of Li = mass of Li / molar mass of Li moles of Li = 12.3 g / 6.94 g/mol The number of moles of N?: moles of N? = mass of N? / molar mass of N? moles of N? = 33.6 g / 28.02 g/mol Now, compare the mole ratios to determine the limiting reactant: Since the mole ratio of Li to and the mole ratio of N? to , we can see that N? is the limiting reactant.