Home /
Expert Answers /
Chemistry /
3-consider-the-two-different-ions-shown-below-which-are-intermediates-formed-in-a-type-of-reaction-pa760
(Solved): 3. Consider the two different ions shown below, which are intermediates formed in a type of reaction ...
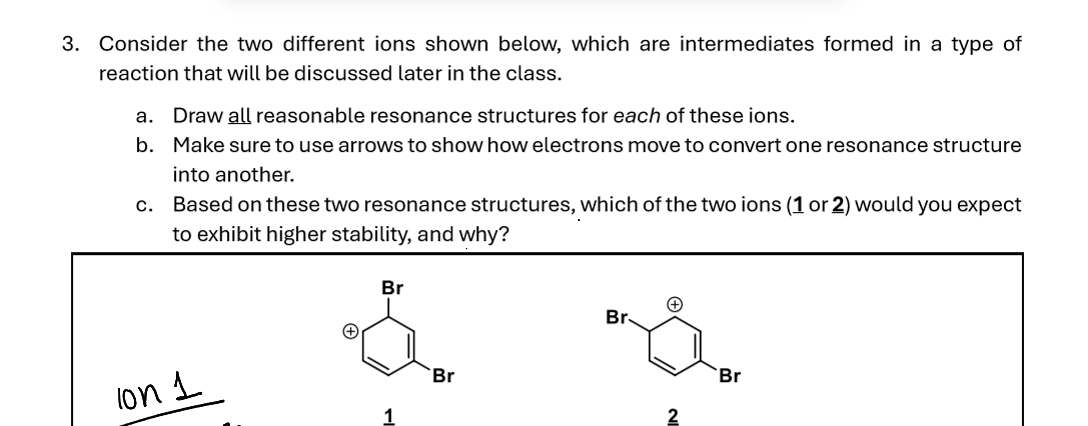
3. Consider the two different ions shown below, which are intermediates formed in a type of reaction that will be discussed later in the class. a. Draw all reasonable resonance structures for each of these ions. b. Make sure to use arrows to show how electrons move to convert one resonance structure into another. c. Based on these two resonance structures, which of the two ions ( \( \underline{\mathbf{1}} \) or \( \underline{\mathbf{2}} \) ) would you expect to exhibit higher stability, and why?