Home /
Expert Answers /
Chemistry /
4-the-isocyanate-ion-cno-has-a-skeleton-of-c-n-o-draw-three-resonance-structures-circle-th-pa235
(Solved): 4. The isocyanate ion (CNO-) has a skeleton of C-N-O. Draw three resonance structures. Circle th ...
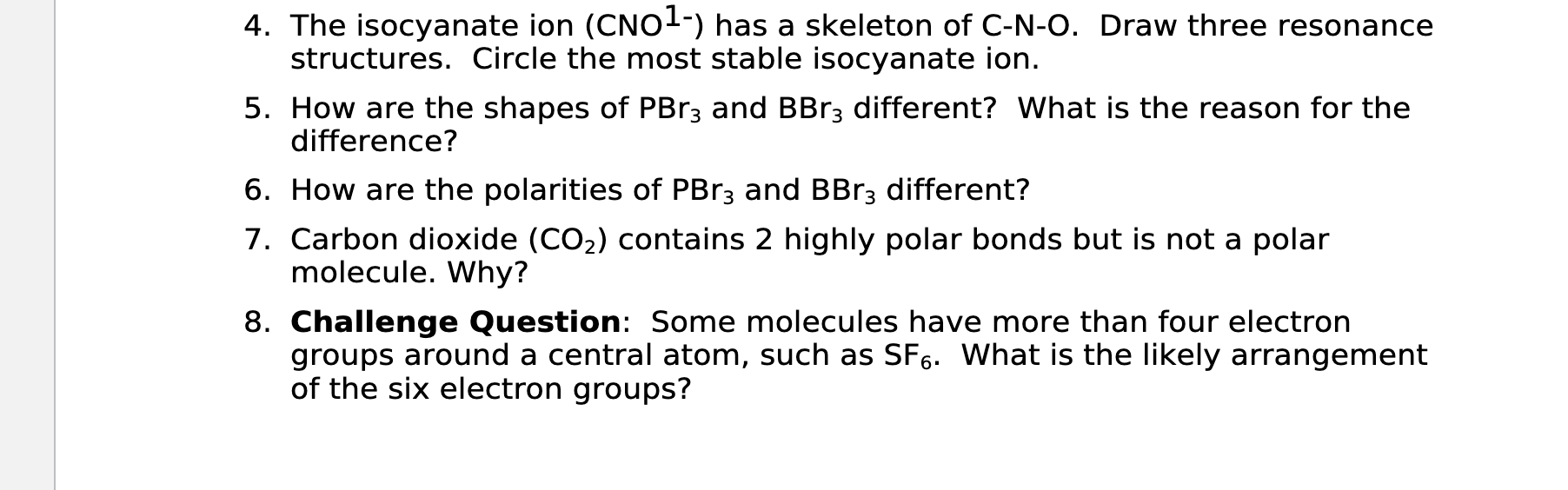
4. The isocyanate ion (CNO¹-) has a skeleton of C-N-O. Draw three resonance structures. Circle the most stable isocyanate ion. 5. How are the shapes of PBr3 and BBr3 different? What is the reason for the difference? 6. How are the polarities of PBr3 and BBr3 different? 7. Carbon dioxide (CO?) contains 2 highly polar bonds but is not a polar molecule. Why? 8. Challenge Question: Some molecules have more than four electron groups around a central atom, such as SF6. What is the likely arrangement of the six electron groups?