Home /
Expert Answers /
Chemical Engineering /
5-5-50-pts-here-is-the-th-zr-phase-diagram-a-5-label-all-2-phase-coexistence-regions-b-5-a-pa339
(Solved): 5-5 [50 pts]: Here is the Th-Zr phase diagram. a. [5] Label ALL 2-phase coexistence regions b. [5] A ...
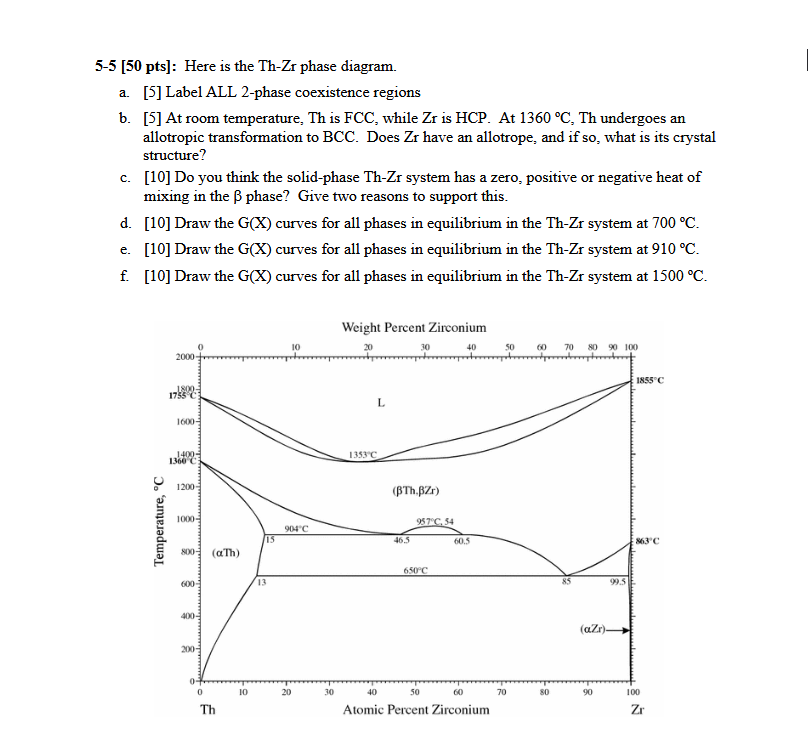
5-5 [50 pts]: Here is the Th-Zr phase diagram.
a. [5] Label ALL 2-phase coexistence regions
b. [5] At room temperature, Th is FCC , while Zr is HCP . At 1360\deg C, Th undergoes an allotropic transformation to BCC . Does Zr have an allotrope, and if so, what is its crystal structure?
c. [10] Do you think the solid-phase Th-Zr system has a zero, positive or negative heat of mixing in the \beta phase? Give two reasons to support this.
d. [10] Draw the G(x) curves for all phases in equilibrium in the Th-Zr system at 700\deg C.
e. [10] Draw the G(x) curves for all phases in equilibrium in the Th-Zr system at 910\deg C.
f. [10] Draw the G(x) curves for all phases in equilibrium in the Th-Zr system at 1500\deg C.