Home /
Expert Answers /
Chemistry /
8-calculate-the-average-atomic-mass-of-chromium-given-the-following-percent-abundances-and-isotop-pa692
(Solved): 8. Calculate the average atomic mass of chromium, given the following percent abundances and isotop ...
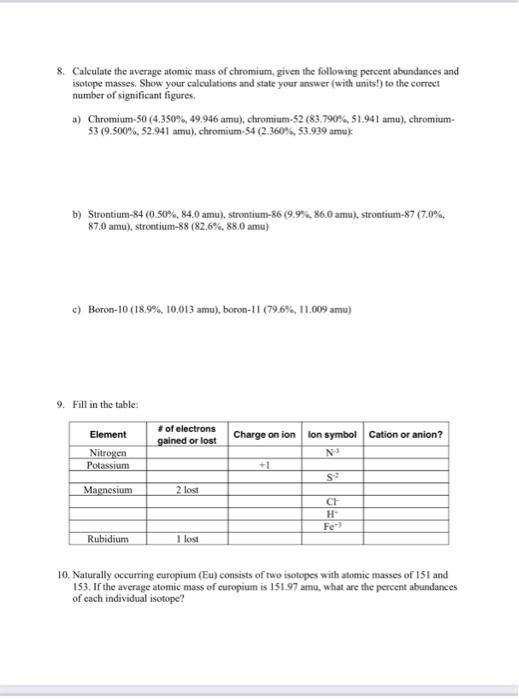
8. Calculate the average atomic mass of chromium, given the following percent abundances and isotope masses. Show your calculations and state your answer (with units!) to the correct number of significant figures. a) Chromium- \( 50(4.350 \%, 49.946 \mathrm{amu}) \), chromium-52 (83.790\%, \( 51.941 \) anu), chromium\( 53(9.500 \%, 52.941 \) amu \( ) \), chromium- \( 54(2.360 \%, 53.939 \mathrm{amu}) \) : b) Strontium-84 (0.50\%, \( 84.0 \) amu), strontium-86 \( (9.954 .86 .0 \) amu), strontium-87 \( (7.0 \% \), \( 87.0 \mathrm{amu}) \), strontium-88 (82.6\%, \( 88.0 \mathrm{amu}) \) c) Boron-10 (18.9\%, 10.013 amu), boron-11 (79.6\%, 11.009 amu) 9. Fill in the table: 10. Naturally occurring curopium (Eu) consists of two isotopes with atomic masses of 151 and 153. If the average atomic mass of europium is \( 151.97 \) amu, what are the pereent abundances of each individual isotope?
Expert Answer
Ans : we use genral formula to calculate average molecular weight, Average molecular weight = M1 × % A1 + M2× % A2 / 100 Where M is mass of isotopes %A id percentage of abundances of isotopes 8 (a) : average atomic weight of chromium = 49.946 ×