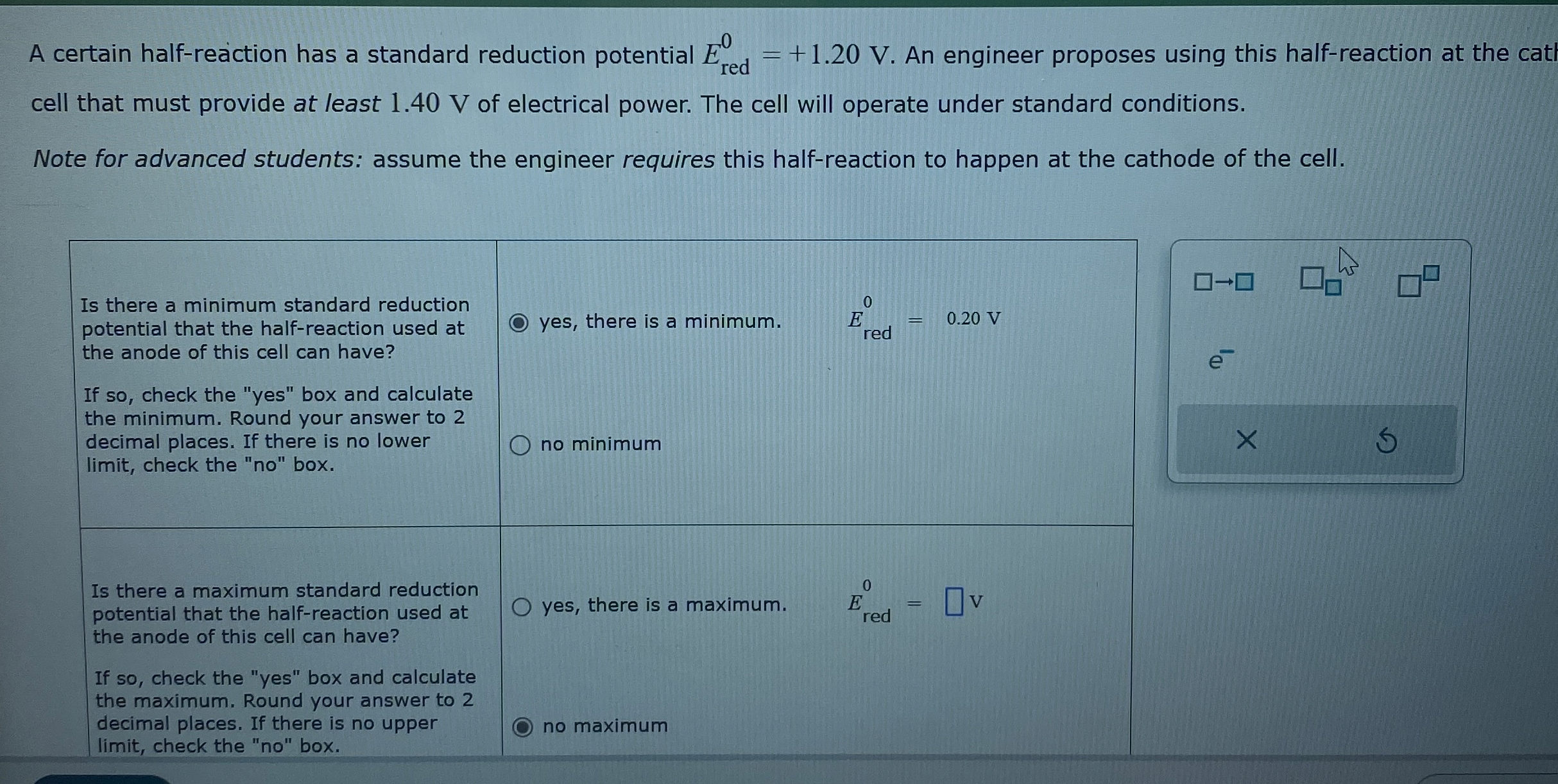
(Solved): A certain half-reaction has a standard reduction potential E_(red)^(0)=+1.20V. An engineer proposes ...
A certain half-reaction has a standard reduction potential
E_(red)^(0)=+1.20V. An engineer proposes using this half-reaction at the catl cell that must provide at least 1.40 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. yes, there is a minimum.
,E_(red )^(0)=0.20Vno minimum Is there a maximum standard reduction potential that the half-reaction used at the anode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. yes, there is a maximum.
E_(red )^(0)=no maximum