Home /
Expert Answers /
Chemical Engineering /
a-define-reaction-selectivity-and-explain-why-selectivity-analysis-is-important-in-chemical-reacto-pa470
(Solved): (a) Define reaction selectivity and explain why selectivity analysis is important in chemical reacto ...
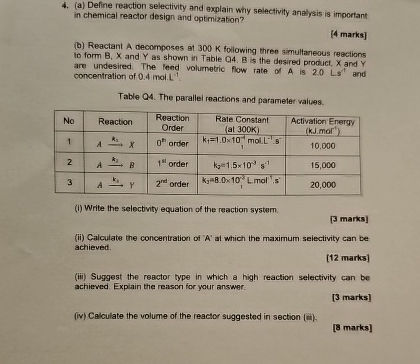
(a) Define reaction selectivity and explain why selectivity analysis is important in chemical reactor design and optimizabion? [4 marks] (b) Reactant
Adecomposes of
300Kfollowing three simullaneous reactions Io form
B,xand
Yas shown in Table
Q4.
Bis the desired product,
xand
Yare undesired. The leed volumetric flow rate of
Ais
20Ls^(-1)and concentration of 0.4 mol.
L^(4)Table Q4. The parallei reactions and parameter values. \table[[No,Reaction,\table[[Reaction],[Order]],\table[[Rate Constant],[(al
300K)]],\table[[Activation Energy],[(k.J.mar')]]],[1,
A->k_(1)x,
0^(hh )order,\table[[
k_(1)=1.0\times 10_(1)^(-1)molL^(-1)*s^(-1)