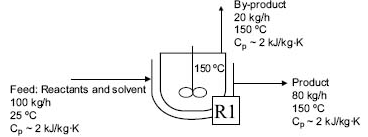
(Solved): A feed to a mixed flow reactor at a pharmaceutical plant (100 kg/h) enters the reaction vessel at 25 ...
A feed to a mixed flow reactor at a pharmaceutical plant (100 kg/h) enters the reaction vessel at 250 C. The reaction takes place at 1500C and the mixture is heated to this temperature within the reactor. Two streams exit the reactor- the product steam at rate of 80 kg/h and the by-product stream at a rate of 20 kg/h (see chart). Both exit streams leave the reactor at temperature of 1500C and they have to be cooled, the product stream to 600C and the by-product stream to 250C. One of the process engineers on site has proposed to save energy by heat integration of the system using heat exchange network. Calculate how much energy can be saved by heat integration in this scheme. Assume that the heat capacity of all the materials involved is 2 kJ/kg and that the minimum temperature approach is 250C.