Home /
Expert Answers /
Chemical Engineering /
a-gaseous-mixture-containing-30mol-co-2-and-70mol-ch-4-is-passed-through-a-joule-thomson-expansi-pa961
(Solved): A gaseous mixture containing 30mol%CO_(2) and 70mol%CH_(4) is passed through a Joule-Thomson expansi ...
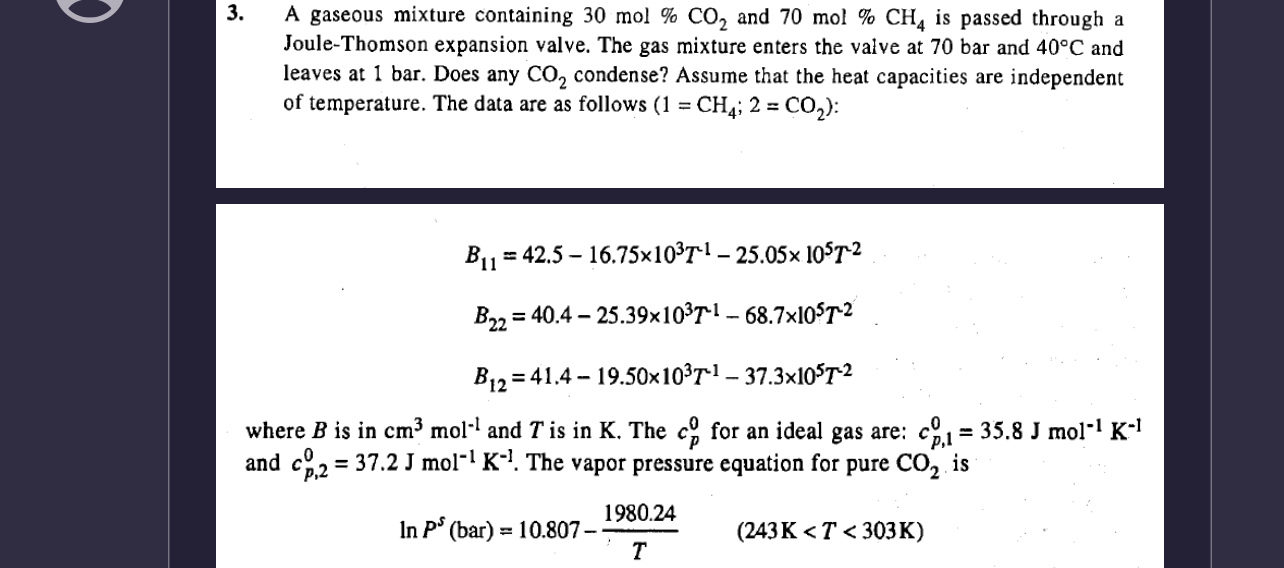
A gaseous mixture containing
30mol%CO_(2)and
70mol%CH_(4)is passed through a Joule-Thomson expansion valve. The gas mixture enters the valve at 70 bar and
40\deg Cand leaves at 1 bar. Does any
CO_(2)condense? Assume that the heat capacities are independent of temperature. The data are as follows