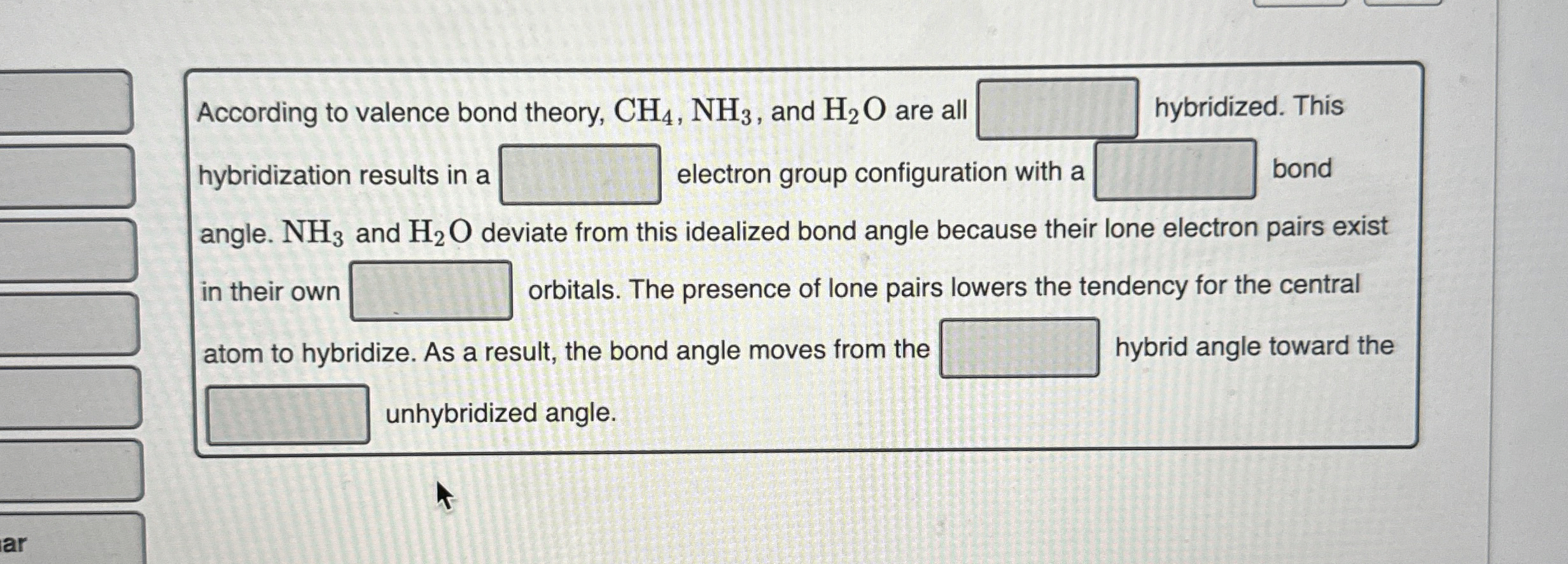
(Solved): According to valence bond theory, CH_(4),NH_(3), and H_(2)O are all hybridized. This hybridization r ...
According to valence bond theory,
CH_(4),NH_(3), and
H_(2)Oare all hybridized. This hybridization results in a electron group configuration with a bond angle.
NH_(3)and
H_(2)Odeviate from this idealized bond angle because their lone electron pairs exist in their own
?orbitals. The presence of lone pairs lowers the tendency for the central atom to hybridize. As a result, the bond angle moves from the hybrid angle toward the unhybridized angle.