Home /
Expert Answers /
Chemistry /
an-alternate-to-an-acetal-for-protection-of-a-ketone-or-aldehyde-is-a-thioacetal-shown-below-whi-pa231
(Solved): An alternate to an acetal for protection of a ketone or aldehyde is a thioacetal, shown below: Whi ...
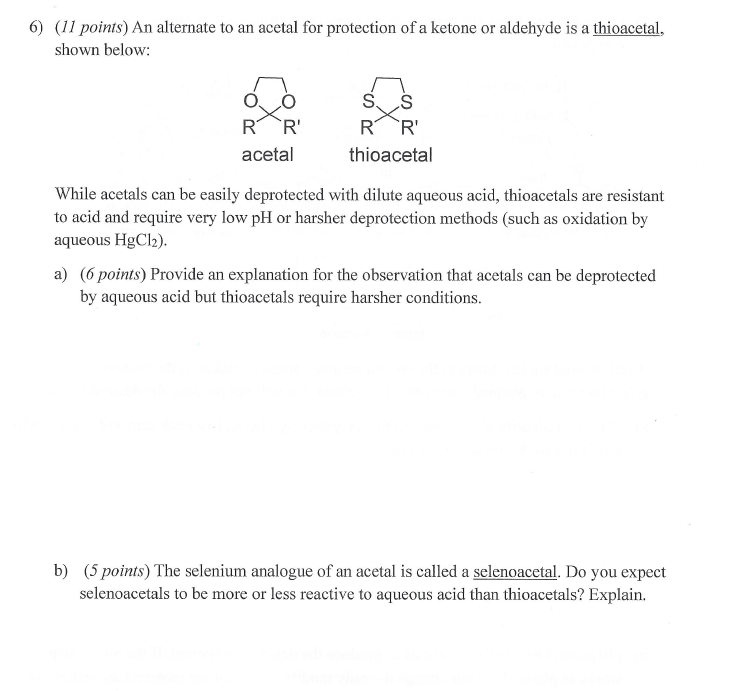
An alternate to an acetal for protection of a ketone or aldehyde is a thioacetal, shown below: While acetals can be easily deprotected with dilute aqueous acid, thioacetals are resistant to acid and require very low pH or harsher deprotection methods (such as oxidation by aqueous \( \mathrm{HgCl}_{2} \) ). a) Provide an explanation for the observation that acetals can be deprotected by aqueous acid but thioacetals require harsher conditions. b) The selenium analogue of an acetal is called a selenoacetal. Do you expect selenoacetals to be more or less reactive to aqueous acid than thioacetals? Explain.