Home /
Expert Answers /
Chemistry /
an-energy-level-scheme-for-the-orbitals-of-second-period-diatomic-molecules-o2-through-ne2-l-pa153
(Solved): An energy level scheme for the orbitals of second period diatomic molecules O2 through Ne2 l ...
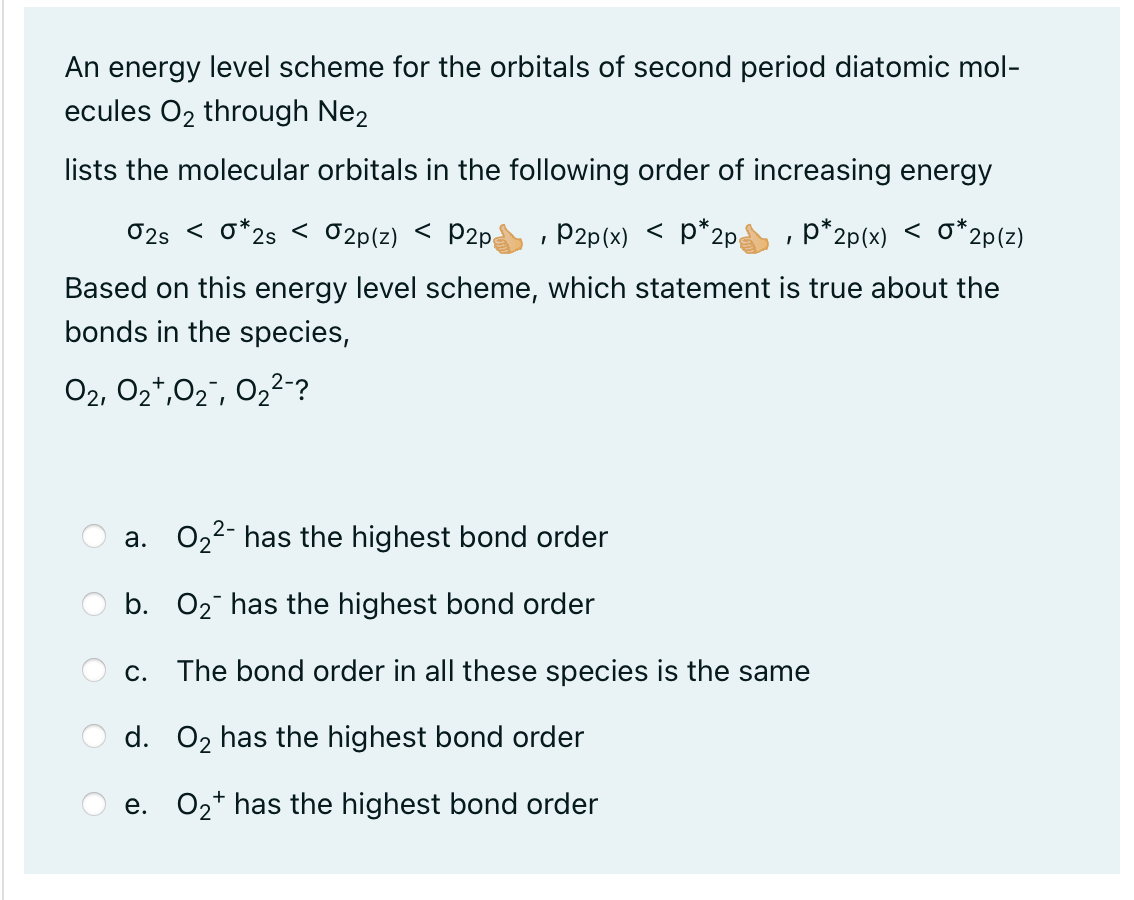
An energy level scheme for the orbitals of second period diatomic molecules through lists the molecular orbitals in the following order of increasing energy Based on this energy level scheme, which statement is true about the bonds in the species, a. has the highest bond order b. has the highest bond order c. The bond order in all these species is the same d. has the highest bond order e. has the highest bond order