Home /
Expert Answers /
Chemistry /
analytical-chemistry-extraction-problem-use-the-formula-provided-on-the-2nd-picture-as-these-a-pa667
(Solved): Analytical Chemistry - Extraction Problem. Use the formula provided on the 2nd picture as these a ...
Analytical Chemistry -? Extraction Problem. Use the formula provided on the 2nd picture as these are only the formulas I learnt.

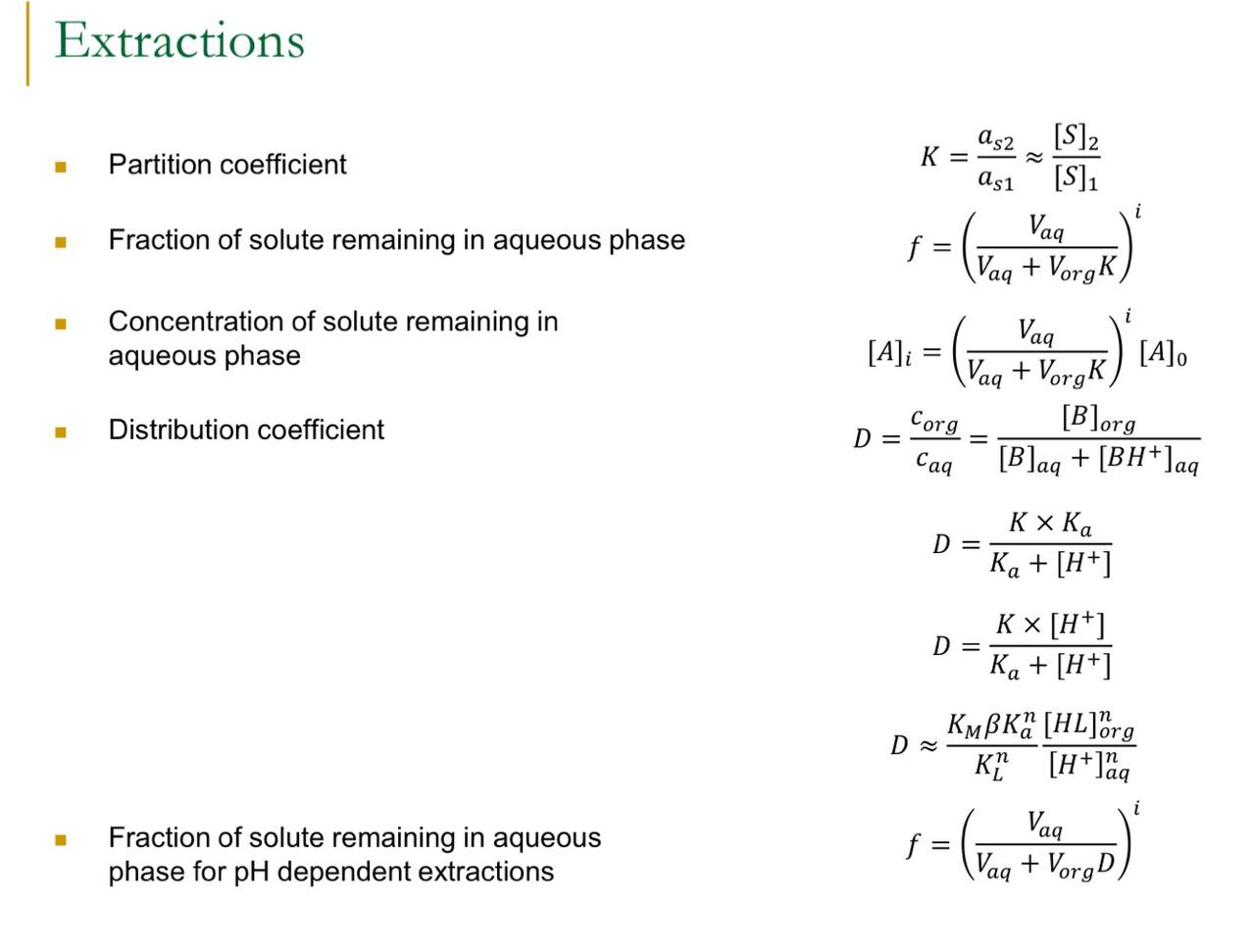
Butanoic acid has a partition coefficient of 3.0 (favouring cyclohexane) when distribut between water and cyclohexane, and acid dissociation constant of of 0 . aqueous butanoic acid is extracted with of cyclohexane at . (a) Determine the distribution coefficient of the acid. (b) Determine the fraction of butanoic acid remaining in water. (c) Find the formal concentration of butanoic acid in cyclohexane. (d) Would you expect the concentration of butanoic acid in cyclohexane to be higher at 4 or ? Justify your answer. (e) What is the minimum volume of cyclohexane required to decrease the concentration of butanoic acid to at least when the butanoic acid is extracted with portions of cyclohexane. Give your answer in units of .
Extractions - Partition coefficient - Fraction of solute remaining in aqueous phase - Concentration of solute remaining in aqueous phase - Distribution coefficient - Fraction of solute remaining in aqueous phase for dependent extractions