Home /
Expert Answers /
Chemistry /
answer-the-following-questions-using-the-mo-electron-configuration-shown-below-for-an-ion-comprised-pa401
(Solved): Answer the following questions using the MO electron configuration shown below for an ion comprised ...
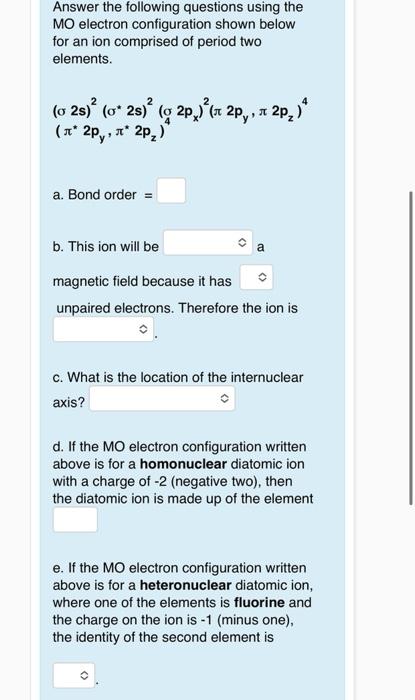
Answer the following questions using the MO electron configuration shown below for an ion comprised of period two elements. \[ \begin{array}{l} (\sigma 2 s)^{2}\left(\sigma^{*} 2 s\right)^{2}\left(\sigma^{2} 2 p_{x}\right)^{2}\left(\pi 2 p_{y}, \pi 2 p_{z}\right)^{4} \\ \left(\pi^{*} 2 p_{y}, \pi^{*} 2 p_{z}\right)^{2} \end{array} \] a. Bond order \( = \) b. This ion will be a magnetic field because it has unpaired electrons. Therefore the ion is c. What is the location of the internuclear axis? d. If the MO electron configuration written above is for a homonuclear diatomic ion with a charge of \( -2 \) (negative two), then the diatomic ion is made up of the element e. If the MO electron configuration written above is for a heteronuclear diatomic ion, where one of the elements is fluorine and the charge on the ion is \( -1 \) (minus one), the identity of the second element is
Expert Answer
as it was not mentioned which questions needed