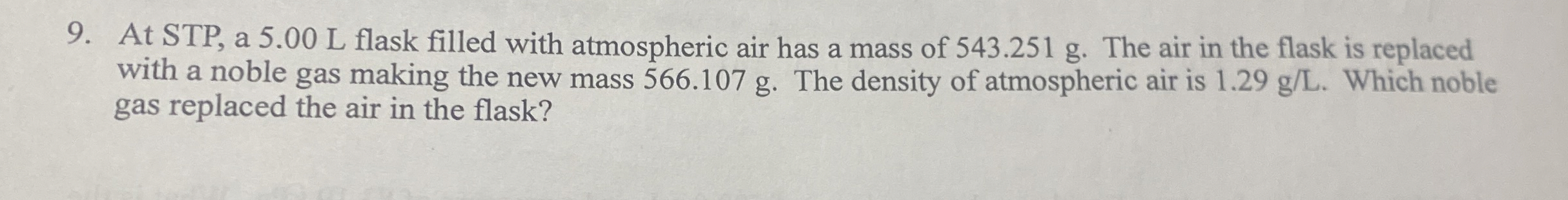Home /
Expert Answers /
Chemistry /
at-stp-a-5-00-l-flask-filled-with-atmospheric-air-has-a-mass-of-543-251-g-the-air-in-the-flask-is-pa546
(Solved): At STP, a 5.00 L flask filled with atmospheric air has a mass of 543.251 g . The air in the flask is ...
At STP, a 5.00 L flask filled with atmospheric air has a mass of 543.251 g . The air in the flask is replaced with a noble gas making the new mass 566.107 g . The density of atmospheric air is
1.29(g)/(L). Which noble gas replaced the air in the flask?