Home /
Expert Answers /
Chemistry /
buffer-distance-0-1-point-graded-the-34-buffer-distance-34-is-defined-as-the-difference-between-pa552
(Solved): Buffer Distance (0)/(1) point (graded) The "buffer distance" is defined as the difference between ...
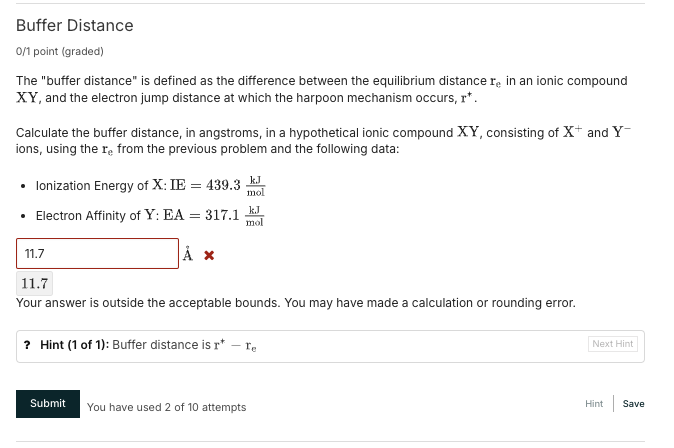
Buffer Distance
(0)/(1)point (graded) The "buffer distance" is defined as the difference between the equilibrium distance
r_(e)in an ionic compound XY, and the electron jump distance at which the harpoon mechanism occurs,
r^(*). Calculate the buffer distance, in angstroms, in a hypothetical ionic compound XY , consisting of
x^(+)and
Y^(-)ions, using the
r_(e)from the previous problemb(which is 2.23 angstroms) and the following data: Ionization Energy of X:
IE=439.3(kJ)/(mol)Electron Affinity of Y: EA
=317.1(kJ)/(mol)Your answer is outside the acceptable bounds. You may have made a calculation or rounding error. ? Hint (1 of 1): Buffer distance is
r^(*)-r_(e)