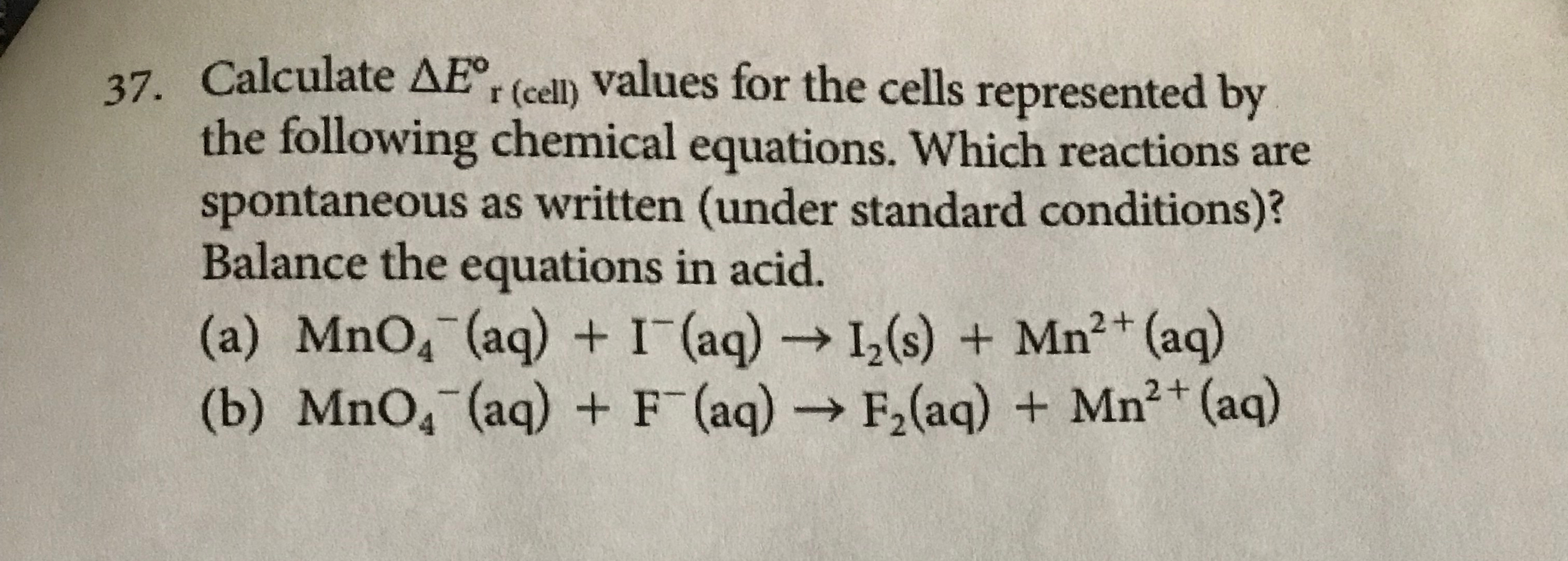Home /
Expert Answers /
Chemistry /
calculate-delta-e-r-cell-deg-values-for-the-cells-represented-by-the-following-chemical-equat-pa513
(Solved): Calculate \Delta E_(r (cell) )\deg values for the cells represented by the following chemical equat ...
Calculate
\Delta E_(r (cell) )\deg values for the cells represented by the following chemical equations. Which reactions are spontaneous as written (under standard conditions)? Balance the equations in acid. (a)
MnO_(4)^(-)(aq)
+I^(-)(aq)->I_(2)(s)+Mn^(2+)(aq) (b)
MnO_(4)^(-)(aq)+F^(-)(aq)->F_(2)(aq)+Mn^(2+)(aq)