Home /
Expert Answers /
Chemistry /
calculate-the-standard-entropy-change-for-the-reaction-at-25-degrees-celcius-mg-oh-2-s-2hcl-g-pa790
(Solved): Calculate the Standard entropy change for the reaction at 25 degrees celcius.Mg(OH)2(s) + 2HCl(g) -- ...
Calculate the Standard entropy change for the reaction at 25 degrees celcius.
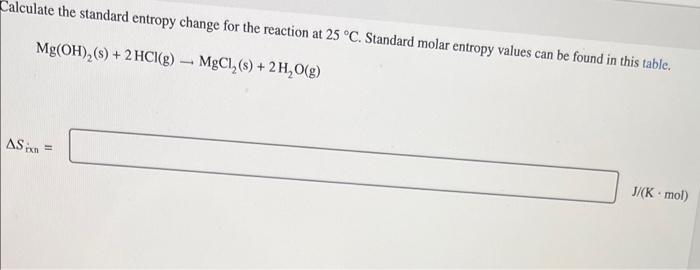
Mg(OH)2(s) + 2HCl(g) ---> MgCl2(s) + 2H2O(g)
Delta Srxn= ? J/(K • mol)

Calculate the standard entropy change for the reaction at . Standard molar entropy values can be found in this table.
Expert Answer
Explanation:First write standard Entropy of all reactants and products at 25oC, and then find the change in