Home /
Expert Answers /
Chemistry /
calculate-the-wavelength-of-a-photon-emitted-by-a-hydrogen-atom-when-its-electron-drops-from-the-n-4-pa987
(Solved): calculate the wavelength of a photon emitted by a hydrogen atom when its electron drops from the n=4 ...
calculate the wavelength of a photon emitted by a hydrogen atom when its electron drops from the n=4 state to the n=3 state
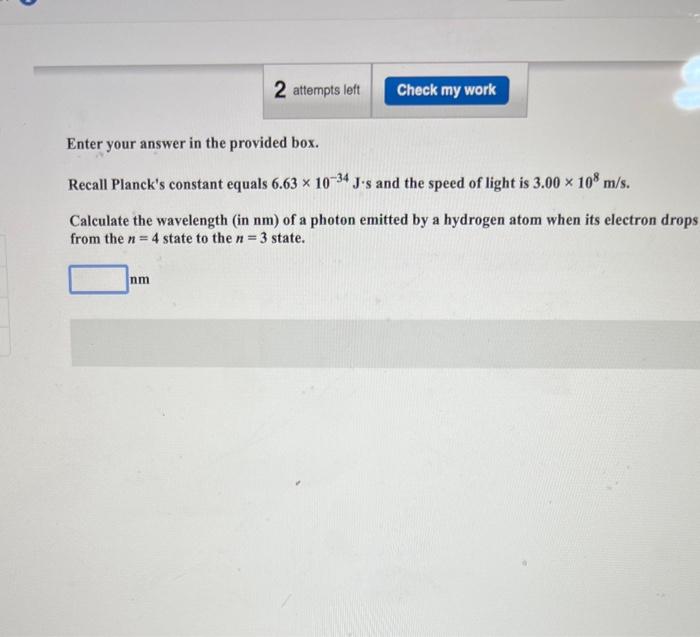

Enter your answer in the provided box. Recall Planck's constant equals \( 6.63 \times 10^{-34} \mathrm{~J} \cdot \mathrm{s} \) and the speed of light is \( 3.00 \times 10^{8} \mathrm{~m} / \mathrm{s} \). Calculate the wavelength (in \( \mathrm{nm} \) ) of a photon emitted by a hydrogen atom when its electron drop from the \( n=4 \) state to the \( n=3 \) state.
Expert Answer
Answer : The required wavelength of photon will be 1876 nm Explanation : From Rydberg's formula, we know that 1 / w = RH [ 1/n21 - 1/n22 ] Wher