Home /
Expert Answers /
Advanced Physics /
carbon-tetrachloride-ccl4-is-diffusing-through-benzene-c6h6-as-the-drawing-illustrate-pa638
(Solved): Carbon tetrachloride (CCl4) is diffusing through benzene (C6H6), as the drawing illustrate ...
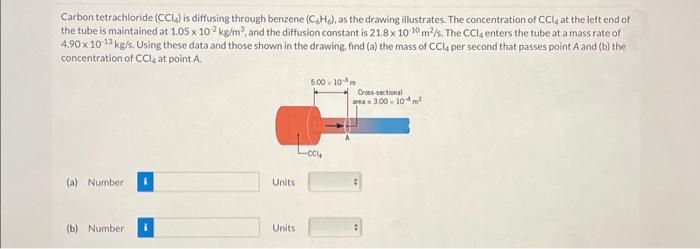
Carbon tetrachloride is diffusing through benzene , as the drawing illustrates. The concentration of at the left end of the tube is maintained at , and the diffusion constant is . The enters the tube at a mass rate of . Using these data and those shown in the drawing. find (a) the mass of per second that passes point and (b) the concentration of at point .
Expert Answer
The given data is as Concentration of CCl4 at the left end 1.05 10-2 kg/m3 The mass rate (m/t) of CCl4 at the left end 4.90 10-13 kg/sDiffusion constant 21.8 10-10 m2/sCross sectional area at point A 3 10-4 m2