Home /
Expert Answers /
Chemical Engineering /
consider-a-daniell-cell-at-25-circ-mathrm-c-with-concentrations-mathrm-b-left-ma-pa647
(Solved): Consider a Daniell cell at \( 25^{\circ} \mathrm{C} \), with concentrations \( \mathrm{b}\left(\ma ...
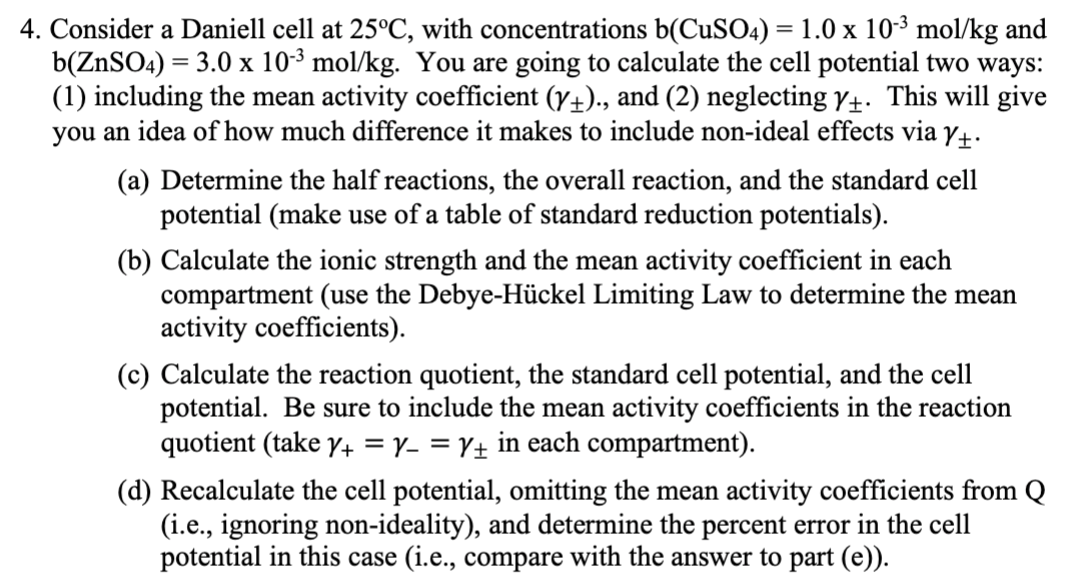
Consider a Daniell cell at \( 25^{\circ} \mathrm{C} \), with concentrations \( \mathrm{b}\left(\mathrm{CuSO}_{4}\right)=1.0 \times 10^{-3} \mathrm{~mol} / \mathrm{kg} \) and \( \mathrm{b}\left(\mathrm{ZnSO}_{4}\right)=3.0 \times 10^{-3} \mathrm{~mol} / \mathrm{kg} \). You are going to calculate the cell potential two ways: (1) including the mean activity coefficient \( \left(\gamma_{\pm}\right) \)., and (2) neglecting \( \gamma_{\pm} \). This will give you an idea of how much difference it makes to include non-ideal effects via \( \gamma_{\pm} \). (a) Determine the half reactions, the overall reaction, and the standard cell potential (make use of a table of standard reduction potentials). (b) Calculate the ionic strength and the mean activity coefficient in each compartment (use the Debye-Hückel Limiting Law to determine the mean activity coefficients). (c) Calculate the reaction quotient, the standard cell potential, and the cell potential. Be sure to include the mean activity coefficients in the reaction quotient (take \( \gamma_{+}=\gamma_{-}=\gamma_{\pm} \)in each compartment). (d) Recalculate the cell potential, omitting the mean activity coefficients from \( \mathrm{Q} \) (i.e., ignoring non-ideality), and determine the percent error in the cell potential in this case (i.e., compare with the answer to part (e)).
Expert Answer
a) Generally the ionic strength of salt is denoted as follows, I=0.5(SIGMA I=1 TO N)mizi^2 Where, I is the ionic strength of any salt. m is the molar concentration of ions i. zi is the number of charge on cations and anions. The ionic strength