Home /
Expert Answers /
Chemistry /
consider-the-dissolution-of-cacl2-cacl2-s-ca2-aq-2cl-aq-h-81-5kj-an-11-5-g-pa144
(Solved): Consider the dissolution of CaCl2 : CaCl2(s)Ca2+(aq)+2Cl(aq)H=81.5kJ An 11.5-g ...
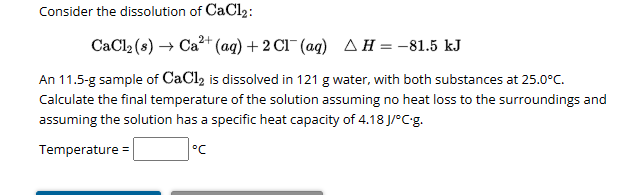
Consider the dissolution of : An 11.5-g sample of is dissolved in water, with both substances at . Calculate the final temperature of the solution assuming no heat loss to the surroundings and assuming the solution has a specific heat capacity of . Temperature