Home /
Expert Answers /
Chemistry /
consider-the-following-reaction-and-kinetic-data-h-2-so-4-g-longrightarrowso-3-g-h-2-o-g-t-pa178
(Solved): Consider the following reaction and kinetic data. H_(2)SO_(4)(g)longrightarrowSO_(3)(g) H_(2)O(g) \t ...
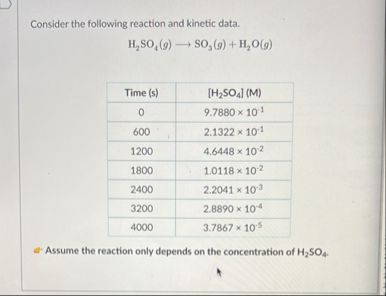
Consider the following reaction and kinetic data.
H_(2)SO_(4)(g)longrightarrowSO_(3)(g) H_(2)O(g)\table[[Time
(s),
[H_(2)SO_(4)](M)What is the order of this reaction? Calculate the rate constant, k, for this reaction. What would be the concentration at 3 minutes?