Home /
Expert Answers /
Chemistry /
consider-the-gas-phase-reaction-between-nitric-oxide-and-oxygen-showing-the-initial-concentrations-pa929
(Solved): Consider the gas-phase reaction between nitric oxide and oxygen showing the initial concentrations ...
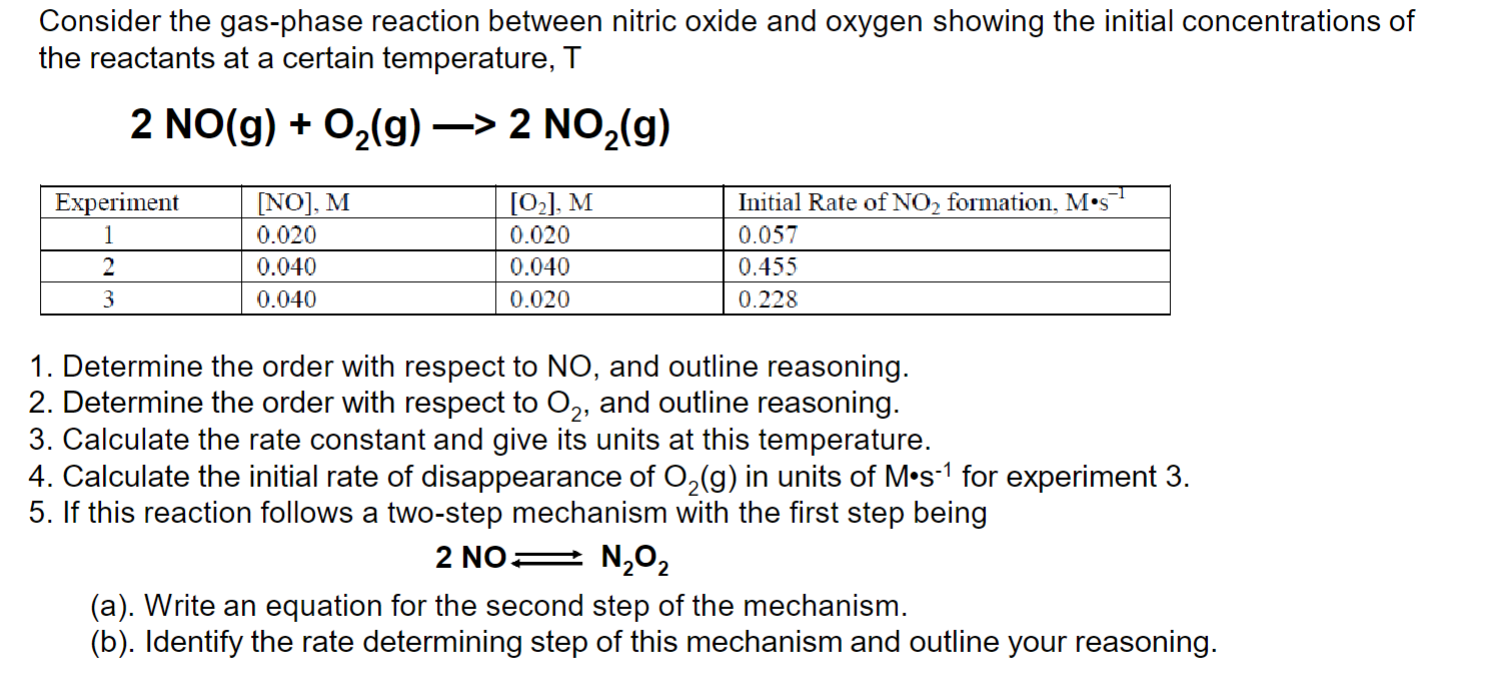
Consider the gas-phase reaction between nitric oxide and oxygen showing the initial concentrations of the reactants at a certain temperature, 1. Determine the order with respect to , and outline reasoning. 2. Determine the order with respect to , and outline reasoning. 3. Calculate the rate constant and give its units at this temperature. 4. Calculate the initial rate of disappearance of in units of for experiment 3. 5. If this reaction follows a two-step mechanism with the first step being (a). Write an equation for the second step of the mechanism. (b). Identify the rate determining step of this mechanism and outline your reasoning.