Home /
Expert Answers /
Chemistry /
consider-this-reaction-3d-g-e-g-2f-g-gt-g-g-2h-g-part-1-of-2-express-the-rate-of-reaction-in-pa478
(Solved): Consider this reaction. 3D(g)+E(g)+2F(g)->G(g)+2H(g) Part 1 of 2 Express the rate of reaction In ...
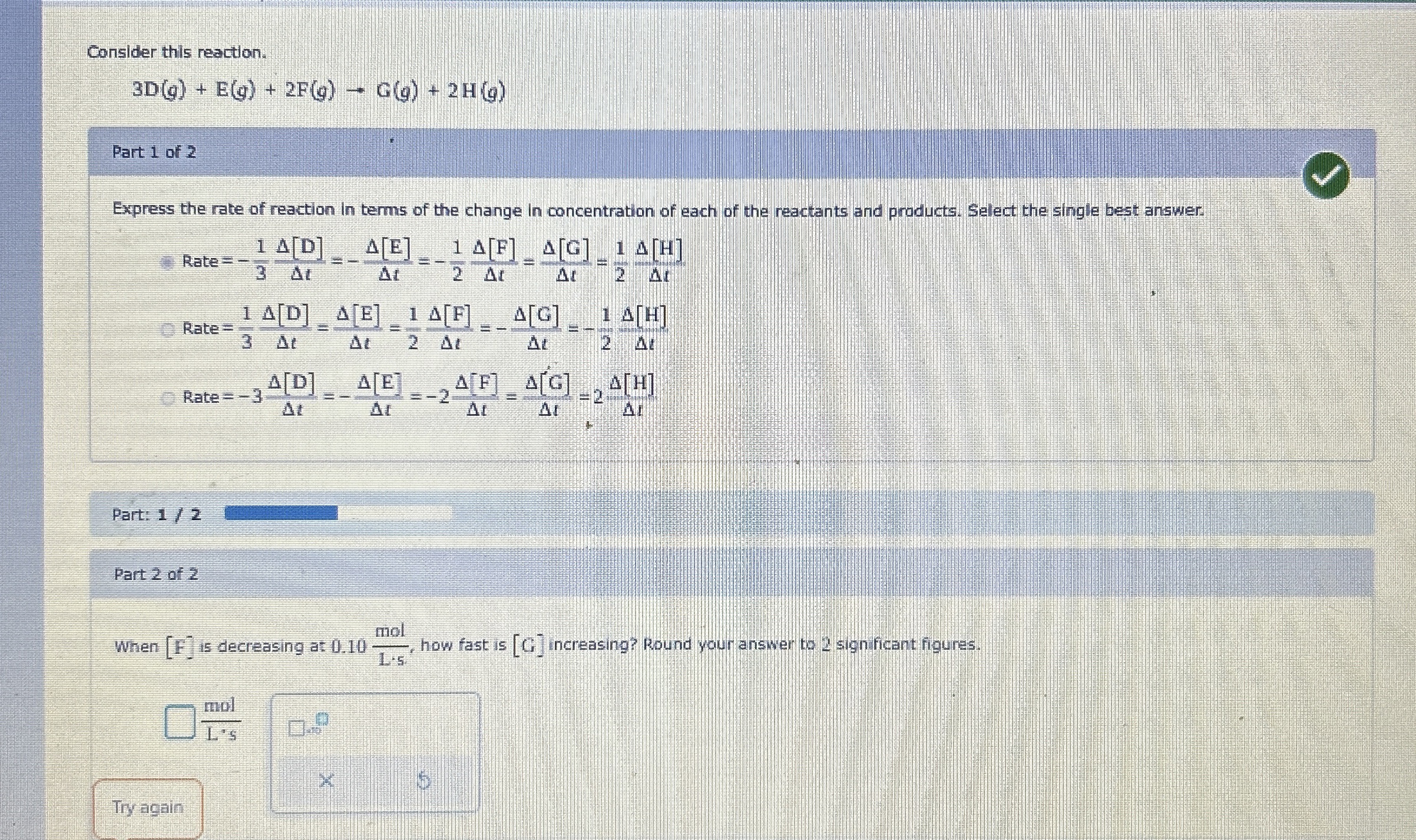
Consider this reaction.
3D(g)+E(g)+2F(g)->G(g)+2H(g)Part 1 of 2 Express the rate of reaction In terms of the change in concentration of each of the reactants and products. Select the single best answer [
:=(1)/(3)(\Delta [D])/(\Delta t)=(\Delta [E])/(\Delta t)=(1)/(2)(\Delta [F])/(\Delta t)=-(\Delta [G])/(\Delta t)=-(1)/(\Delta [H])