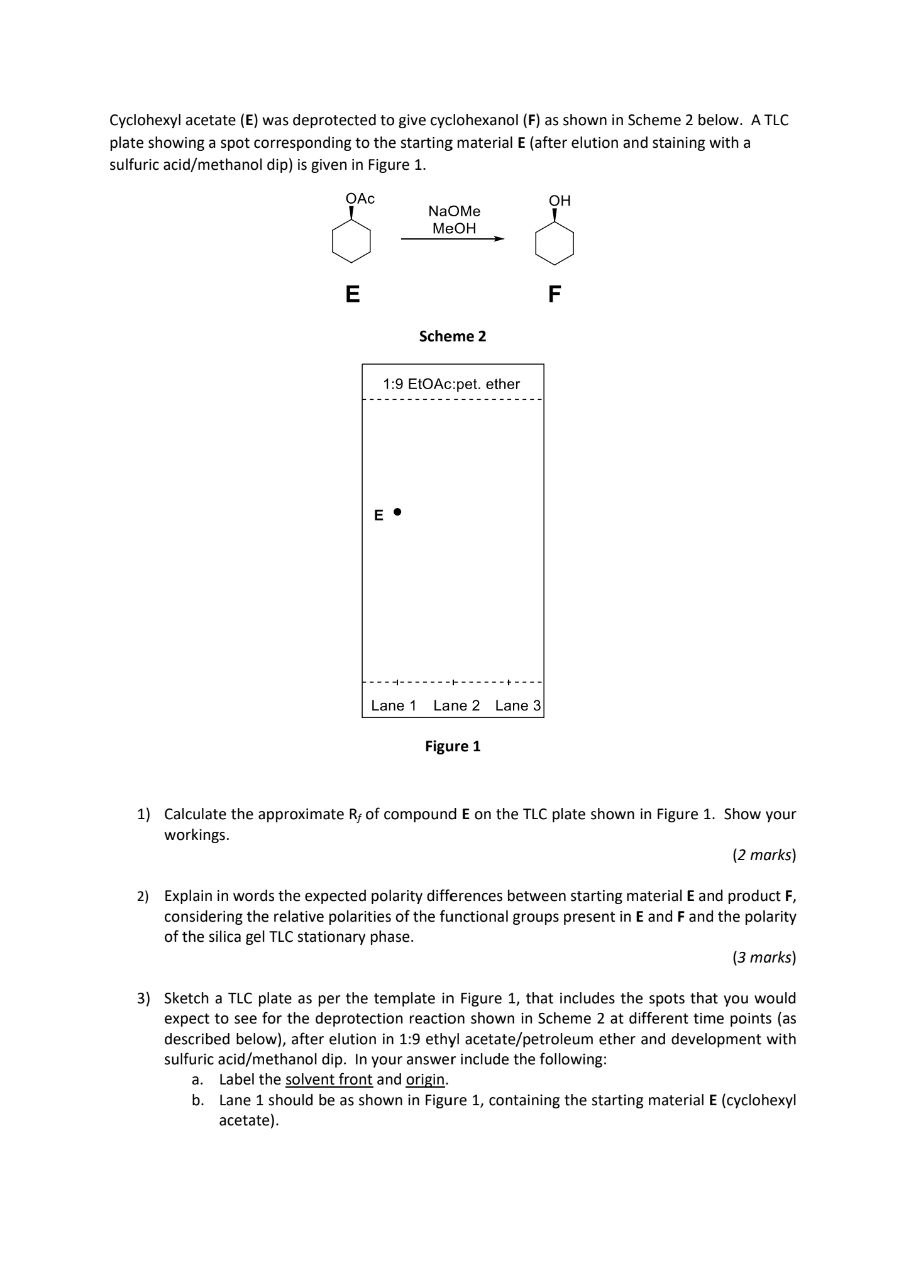
(Solved): Cyclohexyl acetate (E) was deprotected to give cyclohexanol (F) as shown in Scheme 2 below. A TLC p ...
Cyclohexyl acetate (E) was deprotected to give cyclohexanol (F) as shown in Scheme 2 below. A TLC plate showing a spot corresponding to the starting material
E(after elution and staining with a sulfuric acid/methanol dip) is given in Figure 1. Srhomo? Calculate the approximate
R_(f)of compound
Eon the TLC plate shown in Figure 1. Show your workings. Explain in words the expected polarity differences between starting material
Eand product
F, considering the relative polarities of the functional groups present in
Eand
Fand the polarity of the silica gel TLC stationary phase. Sketch a TLC plate as per the template in Figure 1, that includes the spots that you would expect to see for the deprotection reaction shown in Scheme 2 at different time points (as described below), after elution in 1:9 ethyl acetate/petroleum ether and development with sulfuric acid/methanol dip. In your answer include the following: a. Label the solvent front and origin. b. Lane 1 should be as shown in Figure 1, containing the starting material
E(cyclohexyl acetate). c. Lane 2 should show the reaction mixture halfway through the reaction (i.e. a mixture of the starting material
Eand the product
F). d. Lane 3 should show the reaction at completion (i.e. the product
F). [Note that the reactant
NaOMe(sodium methoxide) and the solvent methanol will not show up on TLC so don't try to include them.] If a TLC plate spotted with starting material
Eand product
F(one per lane) is instead eluted with 1:49 ethyl acetate:petroleum ether and developed with sulfuric acid/methanol dip, describe and explain the expected difference(s) in
R_(f)compared to the TLC plate you sketched for question 3 (in which the eluent was 1:9 ethyl acetate:petroleum ether). (4 marks)