Home /
Expert Answers /
Chemistry /
draw-the-lewis-structures-state-the-vsepr-shape-and-the-hybridization-of-the-central-element-final-pa115
(Solved): Draw the Lewis structures, state the VSEPR shape and the hybridization of the central element. Final ...
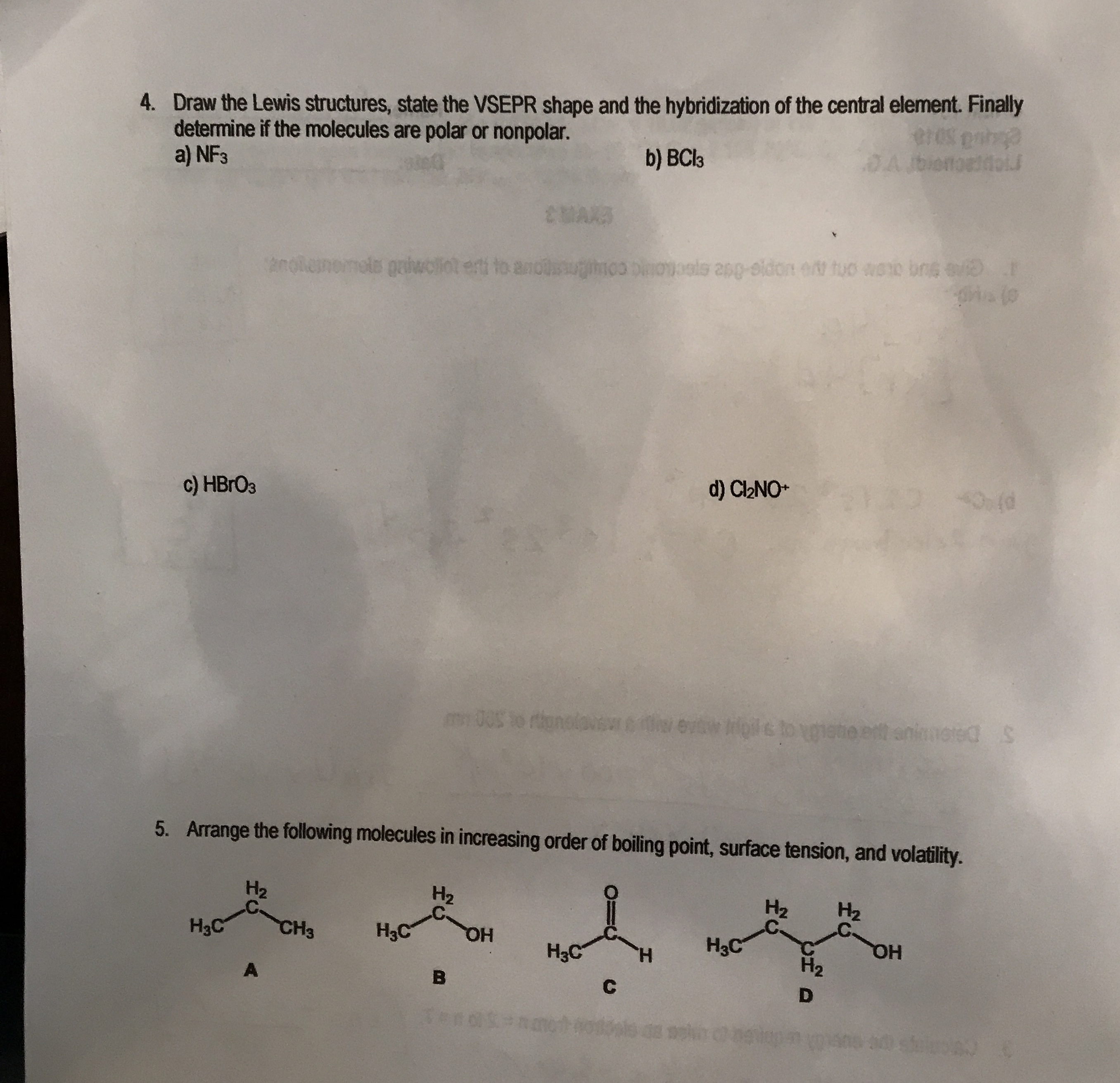
Draw the Lewis structures, state the VSEPR shape and the hybridization of the central element. Finally determine if the molecules are polar or nonpolar. a)
NF_(3)b)
BCl_(3)c)
HBrO_(3)d)
Cl_(2)NO^(+)Arrange the following molecules in increasing order of boiling point, surface tension, and volatility.