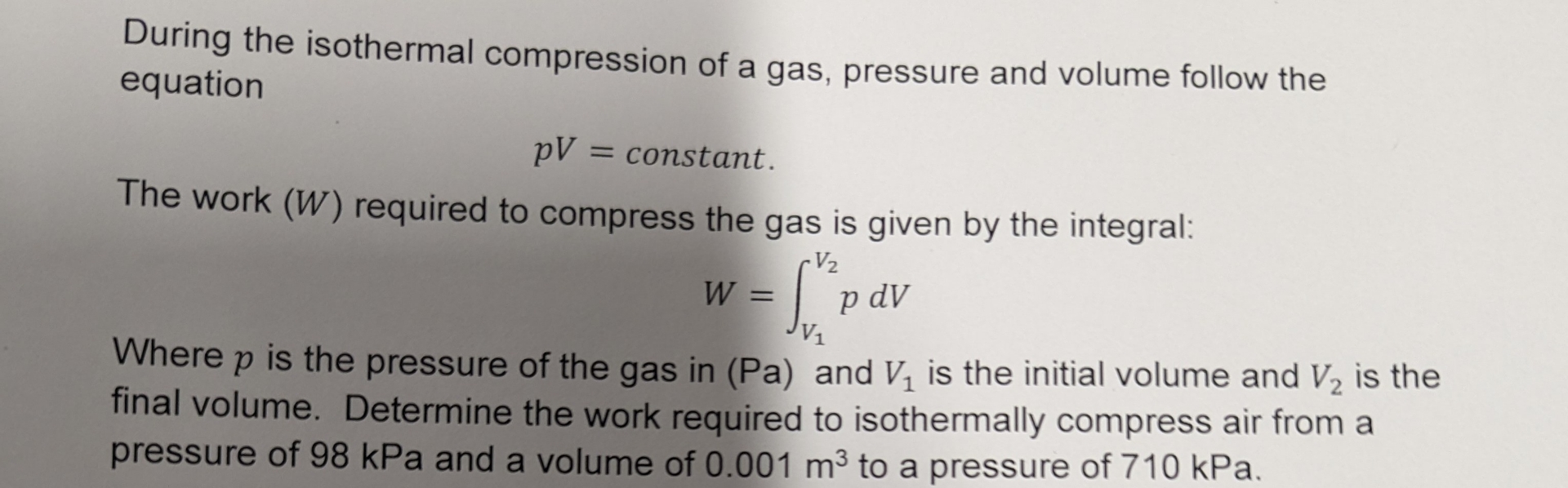Home /
Expert Answers /
Calculus /
during-the-isothermal-compression-of-a-gas-pressure-and-volume-follow-the-equation-pv-constant-th-pa296
(Solved): During the isothermal compression of a gas, pressure and volume follow the equation pV= constant Th ...
During the isothermal compression of a gas, pressure and volume follow the equation
pV= constant The work
(W)required to compress the gas is given by the integral:
W=\int_(V_(1))^(V_(2)) pdVWhere
pis the pressure of the gas in (Pa) and
V_(1)is the initial volume and
V_(2)is the final volume. Determine the work required to isothermally compress air from a pressure of
98kPaand a volume of
0.001m^(3)to a pressure of
710kPa.