Home /
Expert Answers /
Chemistry /
experiment-1-calculating-rate-of-reaction-data-sheet-table-1-10ml-undiluted-1-02-0-iki-a-pa636
(Solved): EXPERIMENT 1: CALCULATING RATE OF REACTION Data Sheet Table 1: 10mL Undiluted (1.02.0%)IKI a ...

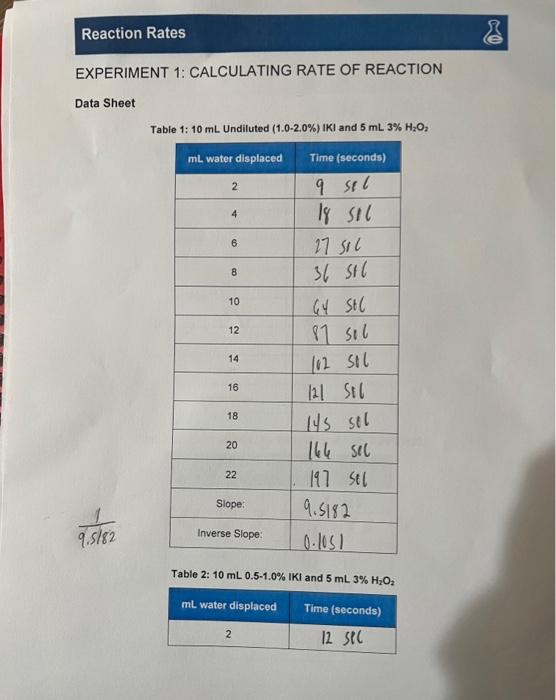
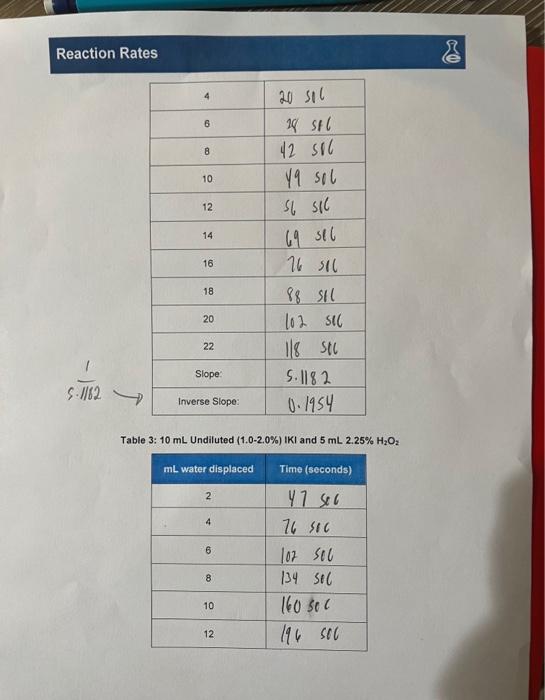
EXPERIMENT 1: CALCULATING RATE OF REACTION Data Sheet Table 1: Undiluted and Table 2: and
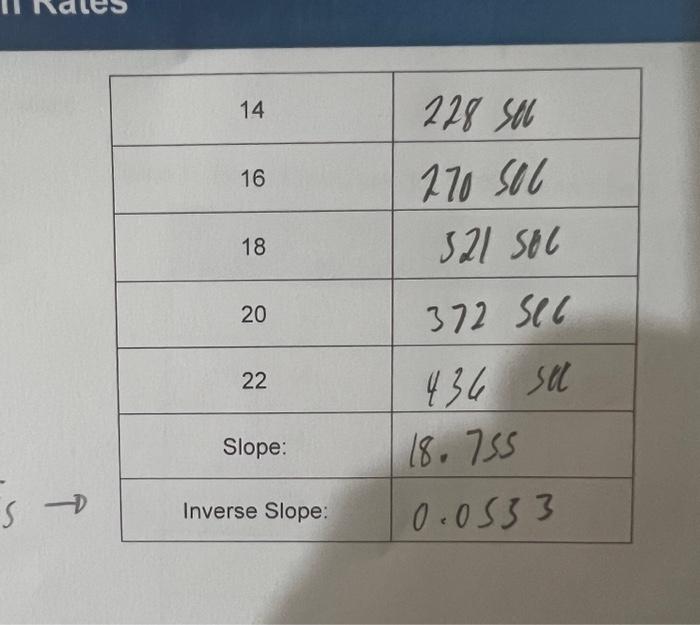
Table 3: Undiluted and
\begin{tabular}{|c|c|} \hline 14 & \\ \hline 16 & \\ \hline 18 & \\ \hline 20 & \\ \hline 22 & \\ \hline Slope: & \\ \hline Inverse Slope: & 0.0533 \\ \hline \end{tabular}
1. Determine the order of the IKI in this reaction. lsk ordir 2. Determine the order of the in this reaction. 3. Calculate the rate law constant. 4. What is the overall rate law? Ist ordir