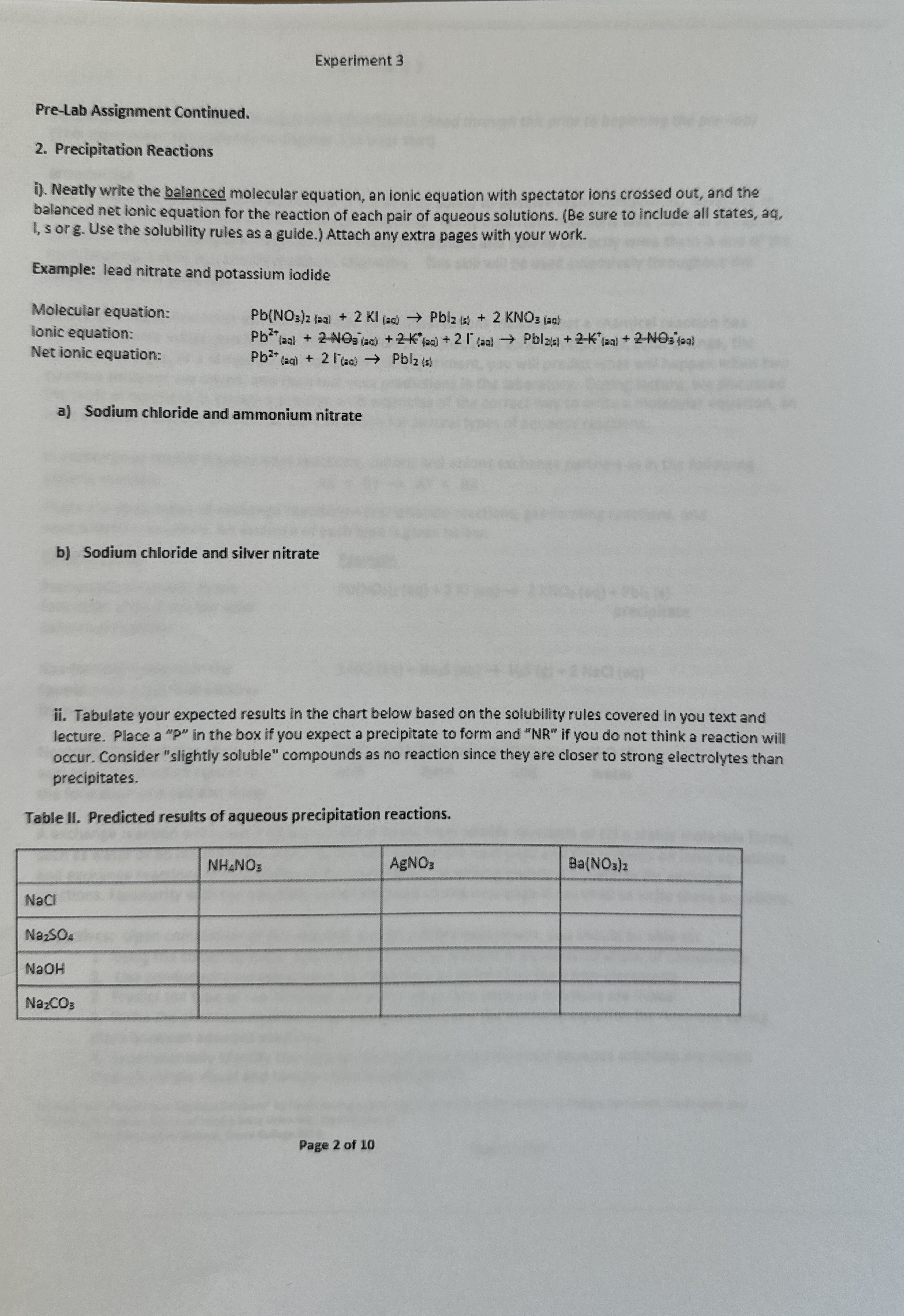
(Solved): Experiment 3 Pre-Lab Assignment Continued. 2. Precipitation Reactions i). Neatly write the balanced ...
Experiment 3 Pre-Lab Assignment Continued. 2. Precipitation Reactions i). Neatly write the balanced molecular equation, an ionic equation with spectator ions crossed out, and the balanced net ionic equation for the reaction of each pair of aqueous solutions. (Be sure to include all states, aq, I, s org. Use the solubility rules as a guide.) Attach any extra pages with your work. Example: lead nitrate and potassium iodide Molecular equation: lonic equation: Net ionic equation: a) Sodium chloride and ammonium nitrate b) Sodium chloride and silver nitrate ii. Tabulate your expected results in the chart below based on the solubility rules covered in you text and lecture. Place a "P" in the box if you expect a precipitate to form and "NR" if you do not think a reaction will occur. Consider "slightly soluble" compounds as no reaction since they are closer to strong electrolytes than precipitates. Table II. Predicted results of aqueous precipitation reactions. \table[[,
NH_(4)NO_(3),
AgNO_(3),
Ba(NO_(3))_(2)