Home /
Expert Answers /
Chemistry /
for-the-aqueous-reaction-dihydroxyacetone-phosphate-glyceraldehyde-3-phosphate-the-standard-chan-pa210
(Solved): For the aqueous reaction dihydroxyacetone phosphate glyceraldehyde-3-phosphate the standard chan ...
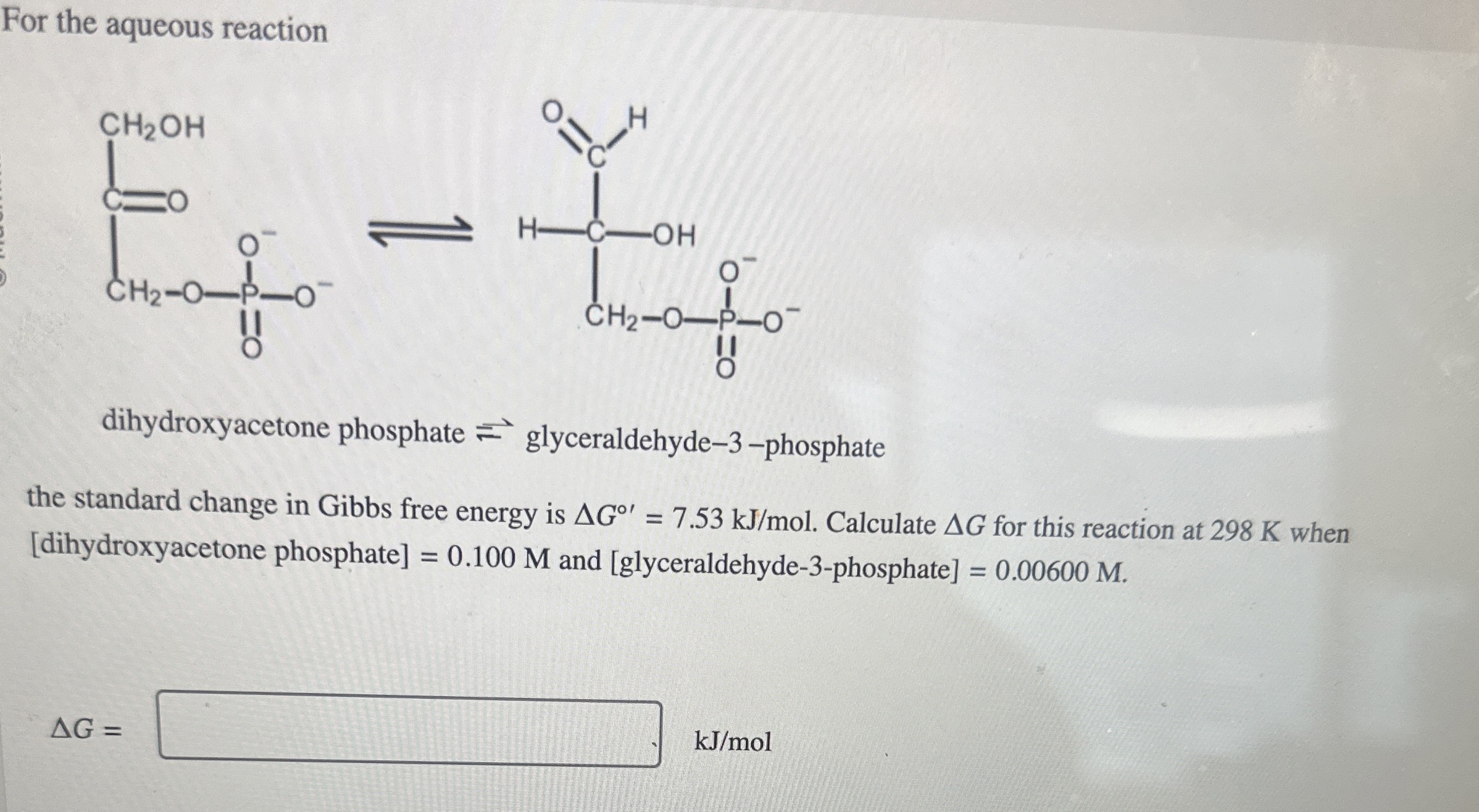
For the aqueous reaction dihydroxyacetone phosphate
?glyceraldehyde-3-phosphate the standard change in Gibbs free energy is
\Delta G^(@')=7.53k(J)/(m)ol. Calculate
\Delta Gfor this reaction at 298 K when [dihydroxyacetone phosphate]
=0.100Mand [glyceraldehyde-3-phosphate]
=0.00600M.
\Delta G=
?
k(J)/(m)ol