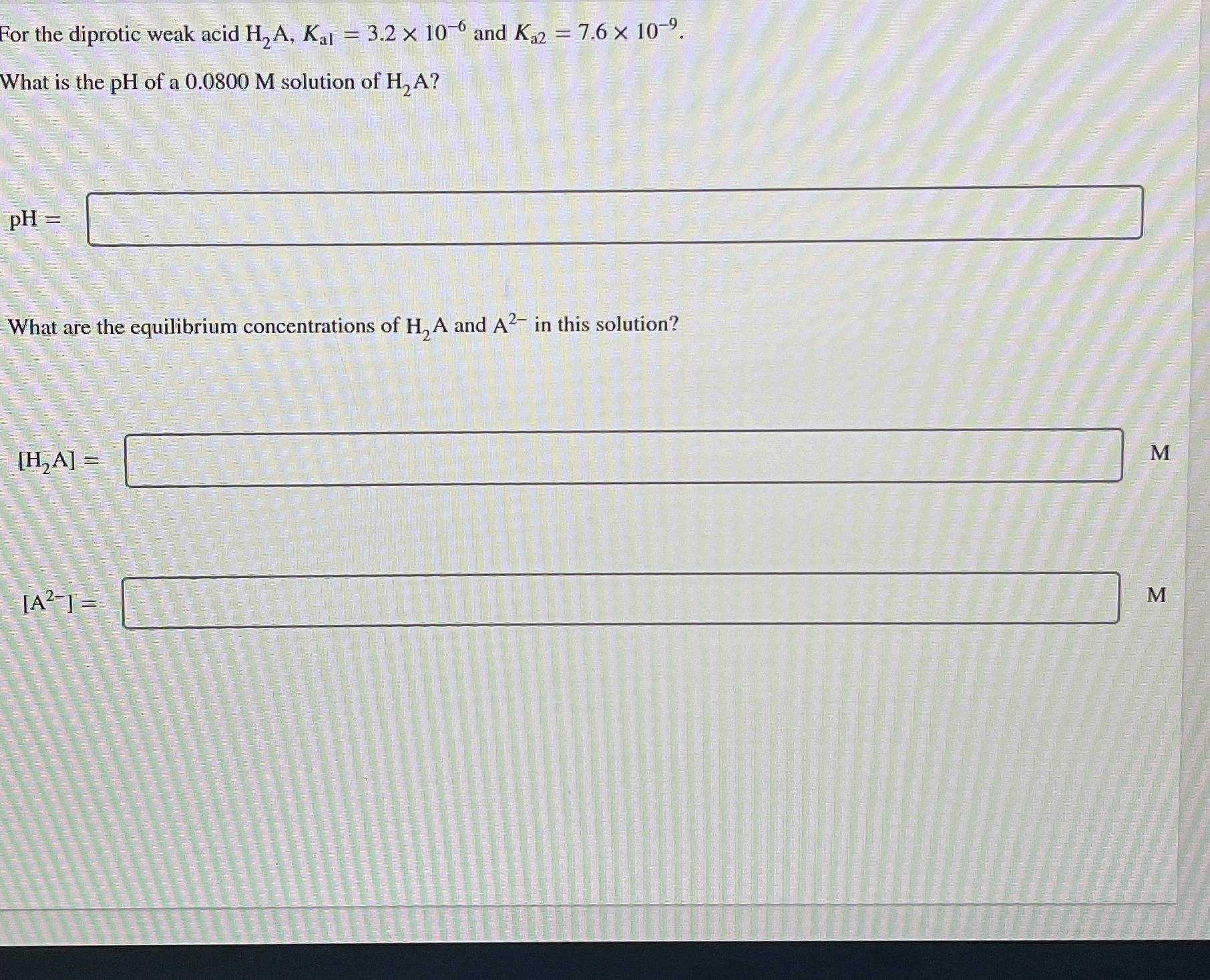
(Solved): For the diprotic weak acid H_(2)A,K_(a1)=3.2\times 10^(-6) and K_(a2)=7.6\times 10^(-9). What is the ...
For the diprotic weak acid
H_(2)A,K_(a1)=3.2\times 10^(-6)and
K_(a2)=7.6\times 10^(-9). What is the pH of a 0.0800 M solution of
H_(2)A?
pH=[?]What are the equilibrium concentrations of
H_(2)Aand
A^(2-)in this solution?
[H_(2)(A)]=
[A^(2-)]=