Home /
Expert Answers /
Chemistry /
for-the-following-reaction-5-80-grams-of-oxygen-gas-are-mixed-with-excess-iron-the-reaction-yields-pa251
(Solved): For the following reaction, 5.80 grams of oxygen gas are mixed with excess iron. The reaction yields ...
For the following reaction, 5.80 grams of oxygen gas are mixed with excess iron. The reaction yields 21.5 grams of iron(II) oxide. What is the theoretical yield of iron(II) oxide ? What is the percent yield for this reaction ? iron (s) + oxygen (g) ? iron(II) oxide (s) % grams
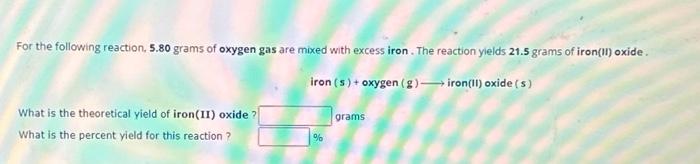
For the following reaction, 5.80 grams of oxygen gas are mixed with excess iron. The reaction yieids 21.5 grams of iron(ii) oxide . What is the theoretical yield of iron(II) oxide? grams What is the percent yield for this reaction ?