Home /
Expert Answers /
Chemistry /
for-the-following-reaction-h-2816kj-mol-heat-6co2-g-6h2o-l-c6h12o6-pa572
(Solved): For the following reaction, H=2816kJ/mol Heat+6CO2(g)+6H2O(l)C6H12O6( ...
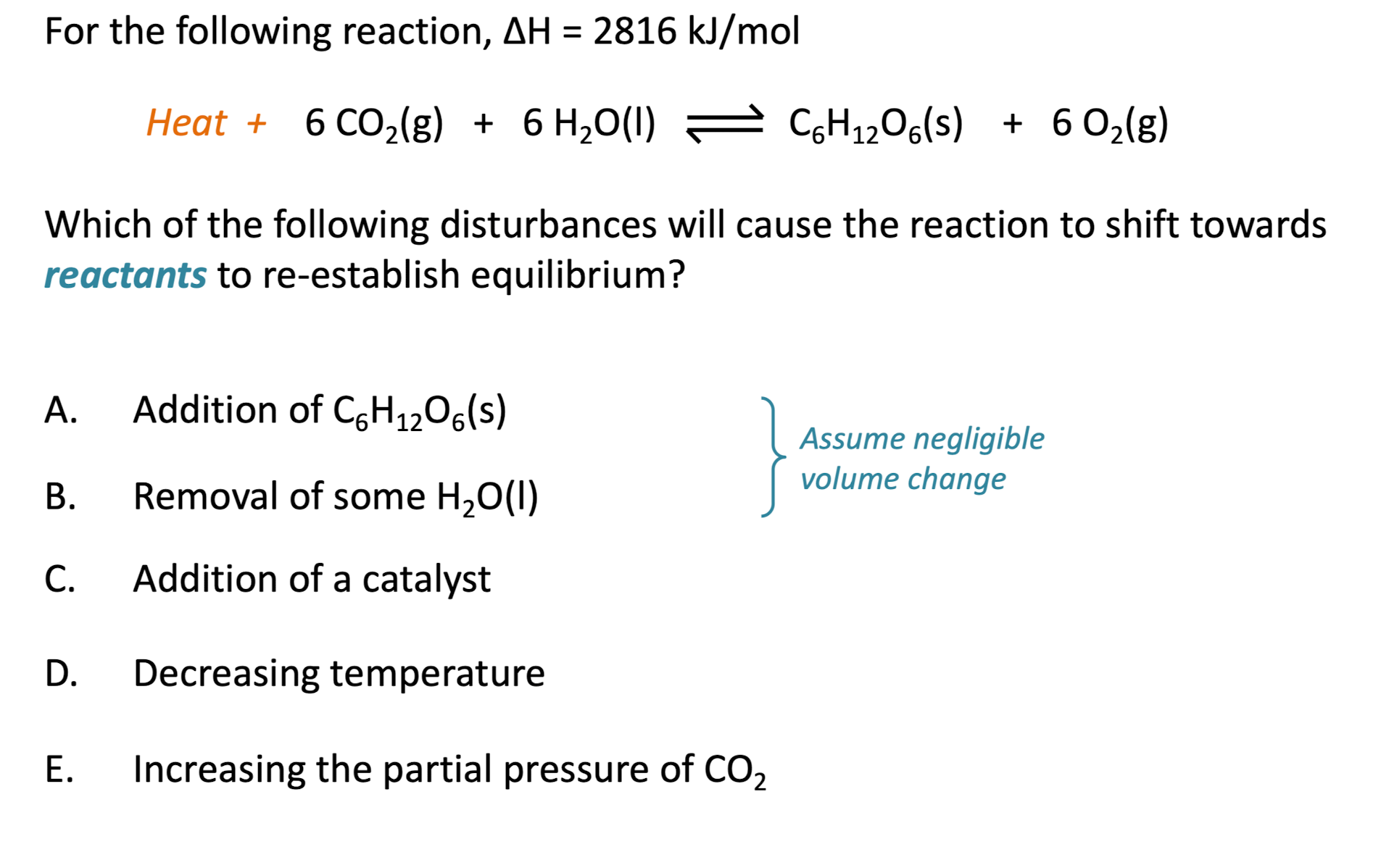
For the following reaction, Which of the following disturbances will cause the reaction to shift towards reactants to re-establish equilibrium? C. Addition of a catalyst D. Decreasing temperature E. Increasing the partial pressure of