Home /
Expert Answers /
Chemistry /
give-the-oxidation-number-of-bromine-in-the-following-part-1-of-4-oxidation-number-of-bromine-in-pa511
(Solved): Give the oxidation number of bromine in the following: Part 1 of 4 Oxidation number of bromine in ...
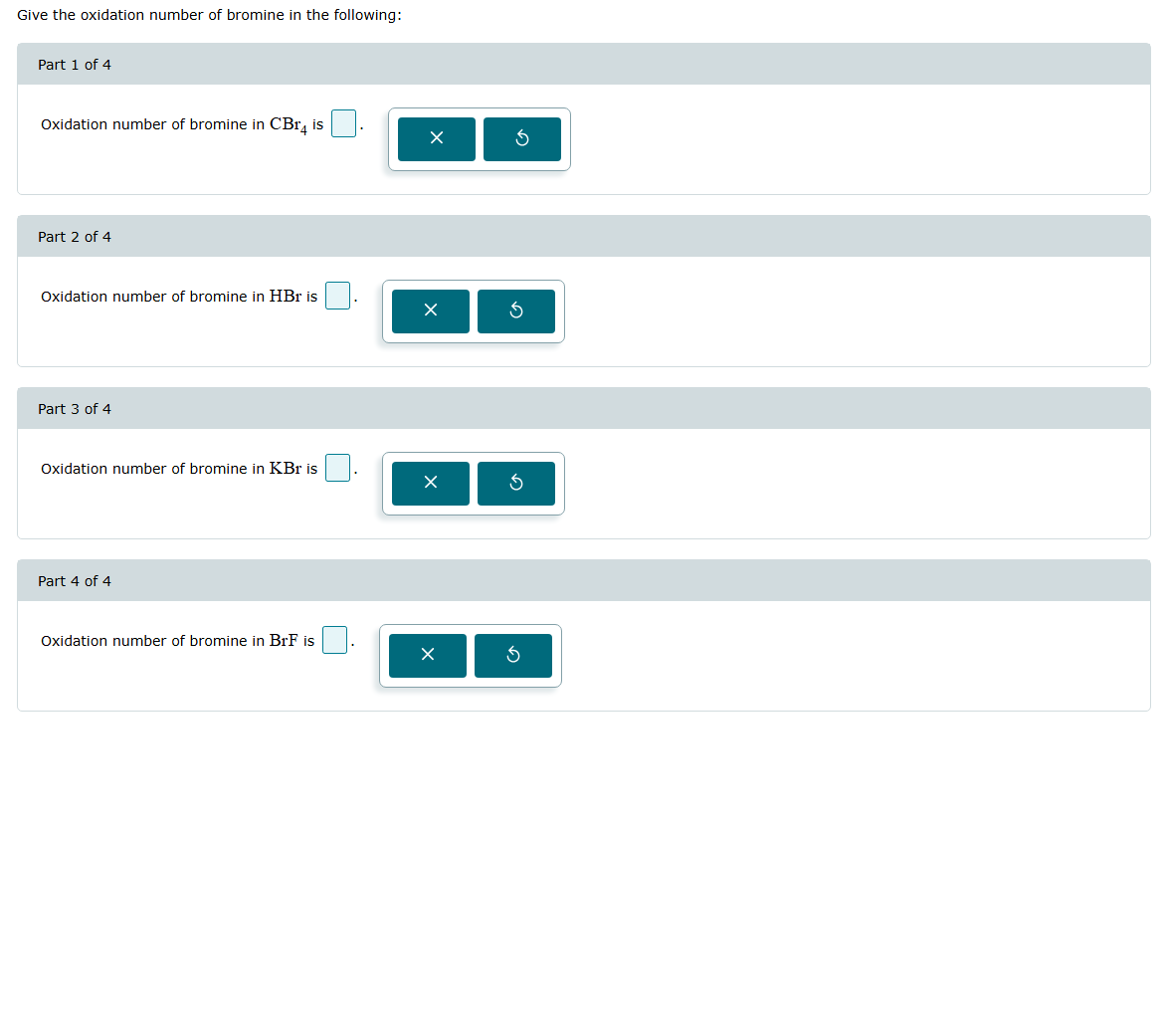
Give the oxidation number of bromine in the following: Part 1 of 4 Oxidation number of bromine in \( \mathrm{CBr}_{4} \) is Part 2 of 4 Oxidation number of bromine in HBr is Part 3 of 4 Oxidation number of bromine in KBr is Part 4 of 4 Oxidation number of bromine in BrF is